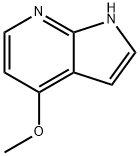
4-METHOXY-7-AZAINDOLE synthesis
- Product Name:4-METHOXY-7-AZAINDOLE
- CAS Number:122379-63-9
- Molecular formula:C8H8N2O
- Molecular Weight:148.16

67-56-1
774 suppliers
$7.29/5ml-f
55052-28-3
483 suppliers
$6.00/1g

122379-63-9
147 suppliers
$5.00/5mg
Yield:122379-63-9 45%
Reaction Conditions:
with sodium at 140; for 44 h;Autoclave;
Steps:
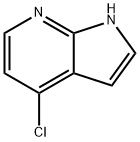
Step 1 - Synthesis of 4-methoxy-1 H-pyrrolo[2,3-b]pyridine (1-120)
To MeOH (600 mL) was added Na (22.6 g, 983 mmol.) in portions over 1 hr, and the mixture stirred to afford a clear solution. Then 4-chloro-1 /-/-pyrrolo[2,3-b]pyridine (1-119) (50 g, 327.69 mmol) was added. The reaction mixture was stirred at 140 °C for 44 hr in a 1 L autoclave. Some yellow solids formed in the reaction mixture, and LCMS showed about 30% of starting material remained. The reaction mixture was concentrated under vacuum to remove MeOH. The residue was diluted into water (200 mL) and extracted with EtOAc/THF ( 2 x 300 mL/50 mL). The extracts were washed with saturated NH4CI (150 mL) and brine (150 mL). The extract was dried and concentrated to give a crude product, which was purified by silica gel chromatography (petroleum ether: EtOAc: THF = 3:1 :0.2 to 1 :1 : 0.2) and re-crystallized from petroleum ether/CH2CI2/EtOH (600 mL/100 mL/10 mL) to give 4-methoxy-1 H-pyrrolo[2,3-b]pyridine (1-120) (22 g, 45%) as a white solid.
References:
PFIZER INC.;JOHNSON, Ted William;RICHARDSON, Paul Francis;COLLINS, Michael Raymond;RICHTER, Daniel Tyler;BURKE, Benjamin Joseph;GAJIWALA, Ketan;NINKOVIC, Sacha;LINTON, Maria Angelica;LE, Phuong Thi Quy;HOFFMAN, Jacqui Elizabeth WO2016/97918, 2016, A1 Location in patent:Page/Page column 91

55052-28-3
483 suppliers
$6.00/1g

122379-63-9
147 suppliers
$5.00/5mg