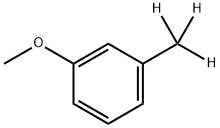
3-METHOXYTOLUENE-A,A,A-D3 synthesis
- Product Name:3-METHOXYTOLUENE-A,A,A-D3
- CAS Number:20369-34-0
- Molecular formula:C8H10O
- Molecular Weight:122.17
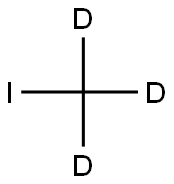
2398-37-0
574 suppliers
$8.00/10g
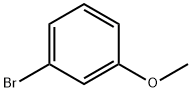
865-50-9
136 suppliers
$25.00/1g

20369-34-0
10 suppliers
$504.23/5MG
Yield:20369-34-0 67.5%
Reaction Conditions:
Stage #1: 3-methoxyphenyl bromidewith n-butyllithium in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2: iodomethane-d3 in tetrahydrofuran at -78 - -10; for 0.5 h;
Steps:
3.1 Step 1 1-methoxy-3-(2H3)methylbenzene as 1iht yellow oil:
To a solution of 1-bromo-3-methoxybenzene (20 g, 106.94 mmol, 1.00 equiv) in THF (200 mL) was added n-BuLi (47.4 mL, 1.10 equiv) at -78 °C under an atmosphere of N2. The resulting solution was stirred for 30 mm at -78 °C. Then CD3I (18.8 g, 1.20 equiv) was added at -78 °C. The resulting solution was stirred for 30 mm at -10 °C. The reaction was then quenched by the addition of NH4C1. The resulting solution was extracted with of ethyl acetate (3 x 80 mL). The organic layers were combined, dried over anhydrous sodium sulfate and concentrated under vacuum to afford 9 g (67.5%) of 1-methoxy-3-(2H3)methylbenzene as light yellow oil.
References:
WO2016/133989,2016,A1 Location in patent:Paragraph 00179