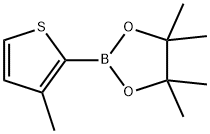
3-METHYLTHIOPHENE-2-BORONIC ACID PINACOL ESTER synthesis
- Product Name:3-METHYLTHIOPHENE-2-BORONIC ACID PINACOL ESTER
- CAS Number:885692-91-1
- Molecular formula:C11H17BO2S
- Molecular Weight:224.13
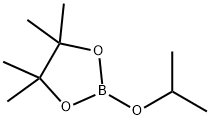
61676-62-8
324 suppliers
$10.00/5g

885692-91-1
128 suppliers
$75.00/100mg
Yield: 53%
Reaction Conditions:
Stage #1:2-bromo-3-methylthiophene with n-butyllithium in tetrahydrofuran;hexane at -40; for 0.5 h;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexane at 20; for 1 h;
Steps:
4.b
To a stirred solution of 2-bromo-3-methythiophene (337 mg, 1.9 mmol) in 8 mL of THF at -40 °C was added n-BuLi (0.8 mL, 2.5 M/hexanes), and the reaction was allowed to stir for 30 min. At this time 2-isopropoxy-4,4,5,5-tetramethyl-[l,3,2]dioxaborolane (775 μL, 3.8 mmol) was added, and the reaction was allowed to warm to ambient temperature, and stirring was continued for 1 h. The reaction was then cooled to 0 C and quenched with satd aq NaHCO3 (10 mL). The mixture was poured into EtOAc (100 mL), washed with H2O (2 x 50 mL), dried (Na2SO4) and concentrated in vacuo. Purification of the residue by silica gel preparative thin layer chromatography (20 % EtOAc-hexanes) afforded 224 mg (53 %) of the title compound as an oil. 1H- NMR (CDCl3; 400 MHz): δ 1.36 (s, 12H), 2.5 (s, 3H), 6.99 (d, IH, J = 4.8 Hz), 7.50 (d, IH, J = 4.8 Hz).
References:
JANSSEN PHARMACEUTICA, N.V. WO2006/47277, 2006, A2 Location in patent:Page/Page column 44
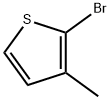
14282-76-9
326 suppliers
$7.00/5g

61676-62-8
324 suppliers
$10.00/5g

885692-91-1
128 suppliers
$75.00/100mg

14282-76-9
326 suppliers
$7.00/5g
185990-03-8
90 suppliers
$17.00/250mg

885692-91-1
128 suppliers
$75.00/100mg
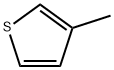
616-44-4
246 suppliers
$18.00/25g
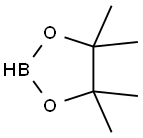
25015-63-8
222 suppliers
$22.39/5G

885692-91-1
128 suppliers
$75.00/100mg

635305-48-5
80 suppliers
$25.00/1g

616-44-4
246 suppliers
$18.00/25g

25015-63-8
222 suppliers
$22.39/5G

885692-91-1
128 suppliers
$75.00/100mg