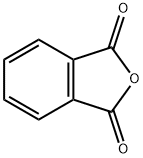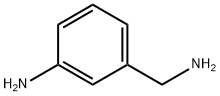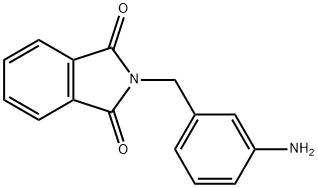
3-N-PHTHALOYLGLYAMINOMETHYL ANILINE synthesis
- Product Name:3-N-PHTHALOYLGLYAMINOMETHYL ANILINE
- CAS Number:77147-14-9
- Molecular formula:C15H12N2O2
- Molecular Weight:252.27
![2-[(3-nitrophenyl)methyl]isoindole-1,3-dione](/CAS/20180527/GIF/21081-63-0.gif)
21081-63-0
3 suppliers
inquiry

77147-14-9
19 suppliers
$315.00/1g
Yield:77147-14-9 62%
Reaction Conditions:
Stage #1: 2-(3-nitrobenzyl)isoindoline-1,3-dionewith tin(II) chloride dihdyrate in ethanol; for 6 h;Reflux;
Stage #2: with water;sodium hydroxide in ethyl acetate; for 0.5 h;Cooling with ice;
Steps:
67.2
14.420 g 2-(3-Nitro-benzy.)-isoindole-1 ,3-dione (obtained in Step 1 , 51.10 mmol) was dissolved in 250 cm3 of ethyl-alcohol and 46.108 g SnCI2 dihydrate (204.35 mmol) was added in portions. The reaction mixture was refluxed for 6 hours. Then the mixture was evaporated under reduced preasure, 300 cm3 of 2N NaOH and 200 cm3 of ethyl-acetate was added and it was stirred for 30 minutes vigrously while it was being cooled in an ice bath. The precipitated solid was filtered off on a Buchner funel and washed well with ethyl-acetate. Filtrate was separated and extracted further three times with 150 - 150 cm3 ethyl-acetate. The combined organic layer was washed with brine, dried over MgS04, decolorized with activated carbon and evaporated to dryness. The residual solid was recrystallized from minimal amount of acetonitrile and air-dried to give the desired product as an off- white solid. Yield: 7.950 g (62%). Ret. time: 2.42 min., (M+H)+ = 253; 1HNMR (DMSO-d6, 300 MHz), δ (ppm): 7.87 (m, 4H), 6.93 (t, J=7.62 Hz, 1 H), 6.42 (m, 3H), 5.04 (bs, 2H), 4.60 (s, 2H).
References:
WO2011/77171,2011,A1 Location in patent:Page/Page column 32
619-23-8
7 suppliers
$10.00/1g

77147-14-9
19 suppliers
$315.00/1g

1074-82-4
468 suppliers
$5.00/25g

77147-14-9
19 suppliers
$315.00/1g
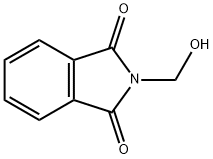
118-29-6
343 suppliers
$5.00/10g

62-53-3
638 suppliers
$10.00/1g

77147-14-9
19 suppliers
$315.00/1g
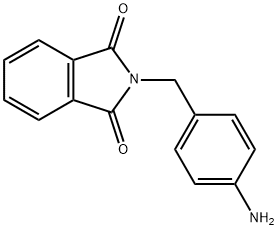
100880-61-3
20 suppliers
$93.00/1/ G