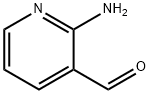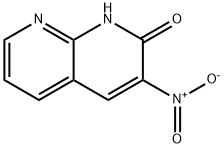
3-Nitro-1,8-naphthyridin-2-ol synthesis
- Product Name:3-Nitro-1,8-naphthyridin-2-ol
- CAS Number:5174-89-0
- Molecular formula:C8H5N3O3
- Molecular Weight:191.14
Yield:5174-89-0 88 %
Reaction Conditions:
with acetic acid in water;Reflux;
Steps:
3.1. Representative procedure for the synthesis of 3-nitro-2(1H)-quinolinones (3)
General procedure: The corresponding substituted 2-aminobenzaldehyde (50 mmol) was dissolved in acetic acid (300 mL) and water (100 mL) and ethyl nitroacetate (75 mmol) was added. The reaction mixture was refluxed until all the starting material was consumed (monitored by HPLC, 16-48 h). Solid was formed in reaction mixture, isolated by filtration and washed with water, dried in vacuo, to give the appropriate 3-nitro-2(1H)-quinolinones.
References:
Molnár, Márk;John, Tamás;Dancsó, András;Nyerges, Miklós [Tetrahedron,2023,vol. 131,art. no. 133225]