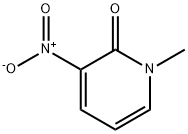
3-nitro-1-methyl-2(1H)-pyridinone synthesis
- Product Name:3-nitro-1-methyl-2(1H)-pyridinone
- CAS Number:32896-91-6
- Molecular formula:C6H6N2O3
- Molecular Weight:154.12
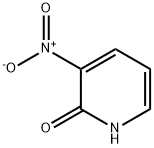
6332-56-5
346 suppliers
$10.00/5g

74-88-4
343 suppliers
$15.00/10g

32896-91-6
23 suppliers
$171.19/1g
Yield:32896-91-6 98%
Reaction Conditions:
Stage #1: 3-nitropyridonewith sodium hydride in tetrahydrofuran; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran at 55; for 16 h;Inert atmosphere;
Steps:
8.2.1. General procedure for alkylation: preparation of compounds 6, 9 and 20
General procedure: Sodium hydride (95%, 1.5 or 2.0 equivalents) was added in portionsto 2-hydroxy-3-nitro pyridine 5 (0.200 g or 2.00 g) or 4-methyl-3-nitropyridin-2(1H)-one 19 (0.663 g) in dry THF (20 or50 mL). The resulting suspension was stirred for 30 min. Methyliodide (1.1 equivalents), benzyl bromide (2.0 equivalents) or ethylbromoacetate (1.1 equivalents) was added dropwise. The resultingyellow suspensionwas heated to 55°C under nitrogen for 16 h (6, 9)or 36 h (20). The red reaction mixture was filtered and the solidthoroughly washed with ethyl acetate. The filtrate was concentratedunder reduced pressure. The crude product was purified bysilica column chromatography (as indicated below; 6, 9, and 20). 8.2.1.1. 1-Methyl-3-nitropyridin-2(1H)-one 6. Chromatography(0-20% ethyl acetate/dichloromethane gradient, with 0.5% Et3N).Yellow solid (0.216 g, 98%): M.p 172-176 °C (dec.) (Lit. [57] 176 °C).FTIR (KBr) ν: 3432, 3085, 1683, 1593, 1540, 1340, 1279, 1111, 915,768 cm-1. 1H NMR (400 MHz, CDCl3) δ: 3.65 (s, 3H, CH3), 6.27 (dd,1H, J = 7.6, 6.8 Hz, H5), 7.70 (dd, 1H, J= 6.4, 2.0 Hz, H6), 8.28 (dd, 1H,J =7.6, 2.0 Hz, H4). 13C NMR (100 MHz, CDCl3) δ: 38.8 (CH3), 103.2(C5), 138.6 (C3, C4), 144.7 (C6), 154.8 (C2). ESI-MS m/z 176.8(M+Na+), 160.8 (M+Li+).
References:
Loughlin, Wendy A.;Jenkins, Ian D.;Karis, N. David;Healy, Peter C. [European Journal of Medicinal Chemistry,2017,vol. 127,p. 341 - 356]

6332-56-5
346 suppliers
$10.00/5g
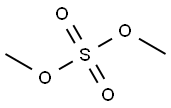
77-78-1
292 suppliers
$22.00/25g

32896-91-6
23 suppliers
$171.19/1g