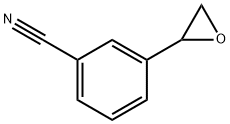
3-(oxiran-2-yl)benzonitrile synthesis
- Product Name:3-(oxiran-2-yl)benzonitrile
- CAS Number:13906-62-2
- Molecular formula:C9H7NO
- Molecular Weight:145.16
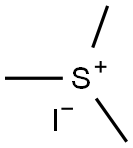
2181-42-2
254 suppliers
$6.00/5g

13906-62-2
16 suppliers
inquiry
Yield:13906-62-2 55%
Reaction Conditions:
with potassium hydroxide in water;acetonitrile at 60; for 15 h;
Steps:
A34.a
A solution of trimethylsulfonium iodide (4.49 g, 22.0 mmol) in acetonitrile (40 ml), KOH (2.64 g, 40.0 mmol) and H2O (0.100 ml) was added at room temperature and stirred at 60 °C for 15 hours. The reaction was quenched with brine, extracted with diethyl ether (100 ml x 2), washed with brine, dried (MgSO4) and evaporated under reduced pressure. The residue was purified by column chromatography on silica (ethyl acetate/hexane, 25/75) to give 3-(oxiran-2-yl)benzonitrile (1.63 g, 55 %, colorless oil).
References:
WO2008/8398,2008,A2 Location in patent:Page/Page column 367
50916-55-7
163 suppliers
$14.14/250mgs:

13906-62-2
16 suppliers
inquiry
5338-96-5
9 suppliers
inquiry

13906-62-2
16 suppliers
inquiry

24964-64-5
317 suppliers
$9.00/1g

13906-62-2
16 suppliers
inquiry