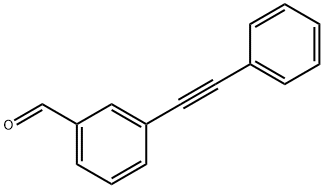
3-PHENYLETHYNYL-BENZALDEHYDE synthesis
- Product Name:3-PHENYLETHYNYL-BENZALDEHYDE
- CAS Number:115021-39-1
- Molecular formula:C15H10O
- Molecular Weight:206.24
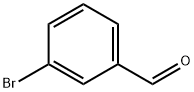
3132-99-8
505 suppliers
$5.00/10g
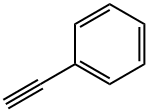
536-74-3
315 suppliers
$10.00/1g

115021-39-1
36 suppliers
$45.00/10mg
Yield:115021-39-1 94%
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane in N,N-dimethyl acetamide at 60; for 24 h;Catalytic behavior;Sonogashira Cross-Coupling;
Steps:
2.6. General procedure for the Sonogashira-Hagihara reaction
General procedure: Aryl halide and a terminal alkyne, with an equivalent molar ratioof 1.0-1.5, were added to a mixture of PdbisindoleSiO2Fe3O4(0.18 mmol, 20 mg) and DABCO (2.0 mmol, 224 mg) in a flask and2 mL DMA was added. The reaction mixture was stirred at 60C foraryl iodides and aryl bromides. The reaction temperature was setto 120C for aryl chlorides and 1 mmol TBAB was also added. Theprogress of the reaction was monitored by gas chromatography.After completion of the reaction, distilled water (2 mL) was addedto the reaction mixture and the crude product was extracted withethyl acetate (3 × 5.0 mL). The crude product was further purifiedby column chromatography using n-hexane and ethyl acetate as eluents.
References:
Gholinejad, Mohammad;Neshat, Abdollah;Zareh, Fatemeh;Nájera, Carmen;Razeghi, Mehran;Khoshnood, Abbas [Applied Catalysis A: General,2016,vol. 525,p. 31 - 40]
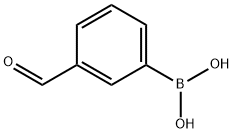
87199-16-4
354 suppliers
$8.00/5g

536-74-3
315 suppliers
$10.00/1g

115021-39-1
36 suppliers
$45.00/10mg
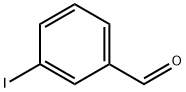
696-41-3
237 suppliers
$7.00/250mg

536-74-3
315 suppliers
$10.00/1g

115021-39-1
36 suppliers
$45.00/10mg
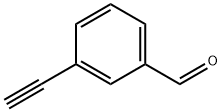
77123-56-9
93 suppliers
$25.00/100mg
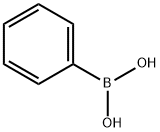
98-80-6
743 suppliers
$5.00/5g

115021-39-1
36 suppliers
$45.00/10mg
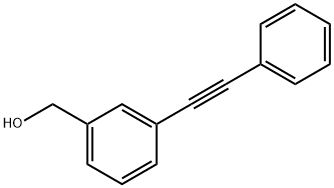
123926-88-5
4 suppliers
inquiry

115021-39-1
36 suppliers
$45.00/10mg