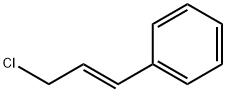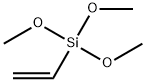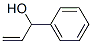
3-Phenylpropene-3-ol synthesis
- Product Name:3-Phenylpropene-3-ol
- CAS Number:4393-06-0
- Molecular formula:C9H10O
- Molecular Weight:134.18
Yield: 73 %Chromat. , 17%
Reaction Conditions:
with Di(2-ethylhexyl)phthalate; pH=6.5 at 30; for 24 h;aq. phosphate buffer;optical yield given as %ee;enantioselective reaction;
Steps:
4.2. General procedure for biotransformation
General procedure: Recombinant E. coli BL21 cells with a dry weight of 0.2 g were resuspended in 10 ml of 100 mM potassium phosphate buffer (pH 6.5) containing 10% (v/v) bis(2-ethylhexyl) phthalate (BEHP) and 8 mg of substrate. The cells used to transform different substrates were from the same batch of culture. The reactions were carried out at 30 °C for 24 h with shaking at 220 rpm and terminated by extraction with diethyl ether. The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure and subjected to reverse phase and chiral HPLC analysis. To determine the specific activity of various substrates, the reactions were terminated after 1 h and the amount of product was analyzed using reverse phase HPLC.For preparative biotransformations, recombinant E. coli BL21 cells with a dry weight of 0.4 g were resuspended in 20 ml of 100 mM potassium phosphate buffer (pH 6.5) containing 10% (v/v) cyclohexane and 30 mg of substrate. The reactions were carried out at 30 °C for 24 h with shaking at 220 rpm, and the products were extracted, concentrated, and subjected to silica gel column chromatography. The purified products were identified with NMR analysis and their specific rotations were measured.
References:
Lin, Hui;Liu, Yan;Wu, Zhong-Liu [Tetrahedron Asymmetry,2011,vol. 22,# 2,p. 134 - 137] Location in patent:experimental part
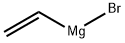
1826-67-1
209 suppliers
$51.29/100ml
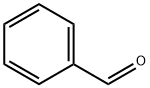
100-52-7
972 suppliers
$17.30/2g
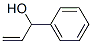
4393-06-0
35 suppliers
inquiry
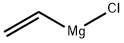
3536-96-7
203 suppliers
$56.60/100ml

100-52-7
972 suppliers
$17.30/2g
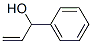
4393-06-0
35 suppliers
inquiry
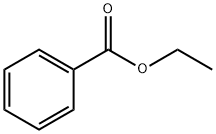
93-89-0
529 suppliers
$13.00/100g
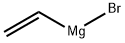
1826-67-1
209 suppliers
$51.29/100ml
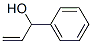
4393-06-0
35 suppliers
inquiry