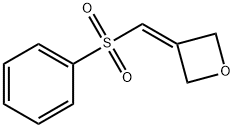
3-(phenylsulfonylMethylene)oxetane synthesis
- Product Name:3-(phenylsulfonylMethylene)oxetane
- CAS Number:1221819-46-0
- Molecular formula:C10H10O3S
- Molecular Weight:210.25
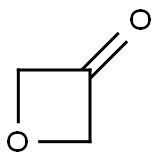
6704-31-0
302 suppliers
$6.00/1g
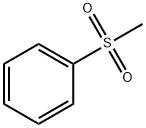
3112-85-4
194 suppliers
$15.00/5g

1221819-46-0
53 suppliers
$30.00/100mg
Yield:1221819-46-0 82%
Reaction Conditions:
Stage #1:methylphenylsulfonate with n-butyllithium in tetrahydrofuran;hexane at 0; for 0.666667 h;
Stage #2: with diethyl chlorophosphate in tetrahydrofuran;hexane for 0.5 h;
Stage #3:oxetan-3-one in tetrahydrofuran;hexane at -78; for 2 h;
Steps:
A-9 3-((Phenylsulfonyl)methylene)oxetane (D A-9)
To a solution of (methylsulfonyl)benzene (2.2 g, 13.9 mmol) in THF (38 mL) at 0 was added n-BuLi (2.5 M in hexanes, 12.2 mL, 30.6 mmol) dropwise over 10 minutes. After the mixture was stirred for 30 min, chlorodiethylphosphonate (2.4 mL, 16.7 mmol) was added dropwise to the reaction. After 30 minutes, a solution of oxetan-3-one (1.0 g, 13.9 mmol) in THF (2 mL) was added dropwise to the reaction mixture at -78 . The reaction mixture was stirred at -78 for 2 hours, then diluted with aqueous NH4Cl (100 mL) and extracted with EtOAc (100 mL x 2) . The combined organic layers were concentrated and the residue was purified by silica gel chromatxography column (petroleum ether/EtOAc = 3/1) to give the title compound (2.4 g, 82%) as a colorless oil. 1H NMR (400 MHz, CDCl3) : δ7.90-7.88 (m, 2H) , 7.68-7.64 (m, 1H) , 7.57 (t, J= 7.6 Hz, 2H) , 6.13-6.11 (m, 1H) , 5.66-5.64 (m, 2H) , 5.30-5.27 (m, 2H).
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;GLAXOSMITHKLINE (CHINA) R&D COMPANY LIMITED;REN, Feng;SANG, Yingxia;XING, Weiqiang;ZHAN, Yang;ZHAO, Baowei WO2018/137619, 2018, A1 Location in patent:Page/Page column 54; 55

6704-31-0
302 suppliers
$6.00/1g
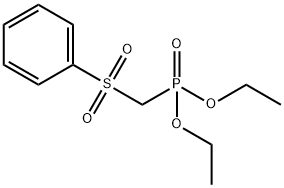
56069-39-7
93 suppliers
inquiry

1221819-46-0
53 suppliers
$30.00/100mg