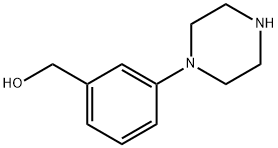
[3-(Piperazin-1-yl)phenyl]methanol synthesis
- Product Name:[3-(Piperazin-1-yl)phenyl]methanol
- CAS Number:795264-41-4
- Molecular formula:C11H16N2O
- Molecular Weight:192.26
![tert-butyl 4-[3-(hydroxymethyl)phenyl]piperazine-1-carboxylate](/CAS/GIF/261925-88-6.gif)
261925-88-6
18 suppliers
$502.88/5MG
![[3-(Piperazin-1-yl)phenyl]methanol](/CAS/20180703/GIF/795264-41-4.gif)
795264-41-4
6 suppliers
$2460.00/1G
Yield:795264-41-4 79%
Reaction Conditions:
with hydrogenchloride;water in ethanol at 20; for 8 h;
Steps:
46.3
This piperazine is prepared from 4- (3-hydroxymethyl- phenyl) -piperazine-1-carboxylic acid tert-butyl ester, itself prepared by reduction in NaBH4 of the corresponding aldehyde, analogous to Bioorg. Med. Chem. Lett. 2003, 13, 3793. In a 250 ml round-bottomed flask, introduce I g of 4- (3- hydroxymethyl-phenyl) -piperazine-1-carboxylic acid tert-butyl ester (4 mmol, 1 eq) and then add 25 ml of ethanol and 25 ml of 30% HCl. Leave for 8 h under agitation at room temperature. The ethanol is then concentrated and the mixture made more basic. Extract with dichloromethane . The organic phase is dried on Na2SO4, filtered, evaporated and purified on silica using a dichloromethane/ethyl acetate gradient. 0.6 g of (3- piperazin-1-yl-phenyl) -methanol is recovered (yield: 79%). 1H NMR (DMSO): 2.80-2.83 (m, 4H, CH2 piperazine), 3.00-3.02 (m, 4H, CH2 piperazine), 4.42 (d, 2H, CH2OH), 5.06 (t, IH, OH), 6.71 (d, IH, H arom), 6.76 (dd, IH, H arom), 6.86 (s, IH, H arom), 7.13 (t, IH, H arom).
References:
WO2008/9741,2008,A1 Location in patent:Page/Page column 54