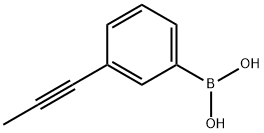
3-(prop-1-ynyl)phenylboronic acid synthesis
- Product Name:3-(prop-1-ynyl)phenylboronic acid
- CAS Number:1189373-19-0
- Molecular formula:C9H9BO2
- Molecular Weight:159.98
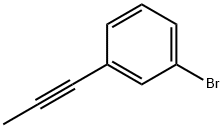
66952-36-1
20 suppliers
$207.75/250mg

1189373-19-0
8 suppliers
inquiry
Yield:1189373-19-0 75%
Reaction Conditions:
Stage #1: 1-bromo-3-(prop-1-yn-1-yl)benzenewith n-butyllithium;Triisopropyl borate in tetrahydrofuran;hexanes;toluene at -78 - 20; for 1.5 h;Inert atmosphere;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;hexanes;toluene at 20; for 0.25 h;
Stage #3: with potassium hydroxide in tetrahydrofuran;hexanes;water;toluene;
Steps:
34
Intermediate 343-(Prop-1-ynyl)phenylboronic acid; n-Butyl lithium (2.5 M in hexanes, 3.7 mL, 9.4 mmol) was added dropwise to a solution of 1-bromo-3-(prop-1-ynyl)benzene (Intermediate 31, 1.66 g, 8.51 mmol) and triisopropyl borate (2.2 mL, 9.4 mmol) in tetrahydrofuran (5 mL) and toluene (15 mL) at -78° C. under an argon atmosphere. The reaction mixture was stirred for 30 min. and then allowed to reach r.t. and stirred for 1 h. The mixture was cooled to -78° C., and 3 M aq. hydrochloric acid was added and the mixture was stirred at r.t. for 15 min. The mixture was basified by addition of solid KOH. 2-methyl-tetrahydrofuran was added under stirring and the obtained solid was collected by filtration affording the title compound 1.0 g (75% yield). 1H NMR (500 MHz, DMSO-d6) δ ppm 2.01 (s, 3H), 6.92-7.03 (m, 1H), 7.09-7.20 (m, 1H), 7.55-7.79 (m, 2H).
References:
US2012/165347,2012,A1 Location in patent:Page/Page column 27
591-18-4
386 suppliers
$5.00/5g

1189373-19-0
8 suppliers
inquiry