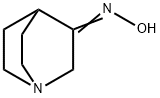
3-QUINUCLIDINONE OXIME synthesis
- Product Name:3-QUINUCLIDINONE OXIME
- CAS Number:35423-17-7
- Molecular formula:C7H12N2O
- Molecular Weight:140.18
1193-65-3
430 suppliers
$19.00/5g

35423-17-7
24 suppliers
$244.00/500mg
Yield:35423-17-7 100%
Reaction Conditions:
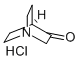
with hydroxylamine hydrochloride;sodium acetate in water at 70; for 2 h;Inert atmosphere;
Steps:
4.1.1.1. Quinuclidine-3-one oxime (34)
To a stirred solution of hydroxylaminehydrochloride (16 g, 230.5 mmol, 1.15 equiv) and sodiumacetate (48.4 g, 589.4 mmol, 3 equiv) in water (150 mL) wasadded dropwise quinuclidine-3-one hydrochloride 33 (32.4 g,200.5 mmol, 1 equiv) dissolved in water (100 mL) and the reactionwas heated at 70 C for 2 h. The mixturewas subsequently cooled tort, and Na2CO3 was added portionwise, bringing the pH of the reactionmixture to 10. The resulting white precipitatewas filtered offunder reduced pressure and the aqueous layer was extracted with amixture of CH2Cl2/i-PrOH 9:1 (3 200 mL). The organic layers weredried over anhydrous Na2SO4, filtered and concentrated underreduced pressure. Both the filtered and extracted solid residueswere combined to afford 34 as a white amorphous solid (28 g, 100%yield); mp 220e222 C. Rf 0.43 (CH2Cl2/MeOH 4:1). 1H NMR(300 MHz, DMSO-d6): d 10.22 (s, 1H), 3.38 (s, 2H), 2.84e2.61 (m,4H), 2.42 (quint, J 3.1 Hz, 1H), 1.78e1.56 (m, 4H). 13C NMR(75 MHz, DMSO-d6): d 162.21, 51.80, 46.73 (2C), 28.32, 26.38 (2C).
References:
Pismataro, Maria Chiara;Horenstein, Nicole A.;Stokes, Clare;Quadri, Marta;De Amici, Marco;Papke, Roger L.;Dallanoce, Clelia [European Journal of Medicinal Chemistry,2020,vol. 205,art. no. 112669]
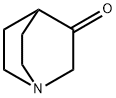
3731-38-2
86 suppliers
inquiry

35423-17-7
24 suppliers
$244.00/500mg
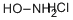
5470-11-1
757 suppliers
$10.00/5G

1193-65-3
430 suppliers
$19.00/5g

35423-17-7
24 suppliers
$244.00/500mg
76883-37-9
25 suppliers
$45.00/10mg

35423-17-7
24 suppliers
$244.00/500mg