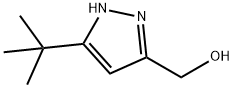
(3-(tert-butyl)-1H-pyrazol-5-yl)methanol synthesis
- Product Name:(3-(tert-butyl)-1H-pyrazol-5-yl)methanol
- CAS Number:493038-53-2
- Molecular formula:C8H14N2O
- Molecular Weight:154.21
916791-97-4
21 suppliers
$30.00/20mg

493038-53-2
11 suppliers
inquiry
Yield:-
Reaction Conditions:
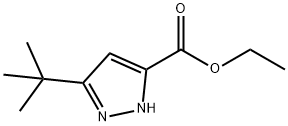
Stage #1: ethyl 3-(tert-butyl)-1H-pyrazole-5-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 1 h;
Stage #2: with sodium sulfate in tetrahydrofuran at 20; for 0.5 h;
Steps:
66
To a solution of ethyl 3-tert-butyl-1H-pyrazole-5-carboxylate (4.0 g, 20.4 mmol) in tetrahydrofuran (50 mL) was added lithium aluminum hydride (800 mg, 21.1 mmol) by small portions at 0°C, and the mixture was allowed to warm to room temperature and stirred for 1 hr. Sodium sulfate decahydrate (7.6 g) was added to the reaction mixture, and the mixture was stirred at room temperature for 30 min. The insoluble material was filtered off. The filtrate was concentrated and the residue was dissolved in tetrahydrofuran (100 mL). Manganese dioxide (10.0 g) was added, and the mixture was stirred at room temperature for 3 hr. The insoluble material was filtered off, and the filtrate was concentrated to give the title compound (2.13 g, yield 69%) as colorless crystals. 1H NMR (CDCl3) δ: 1.37(9H, s), 6.65(1H, s), 9.95(1H, s).
References:
EP1731505,2006,A1 Location in patent:Page/Page column 50
75-97-8
233 suppliers
$10.00/1g

493038-53-2
11 suppliers
inquiry