
2-(4-[(4-METHYLPHENYL)SULFONYL]PIPERAZINO)-1,3-BENZOTHIAZOLE synthesis
- Product Name:2-(4-[(4-METHYLPHENYL)SULFONYL]PIPERAZINO)-1,3-BENZOTHIAZOLE
- CAS Number:309285-78-7
- Molecular formula:C18H19N3O2S2
- Molecular Weight:373.49

98-59-9
588 suppliers
$9.00/5g

55745-83-0
80 suppliers
$49.00/200μmol
![2-(4-[(4-METHYLPHENYL)SULFONYL]PIPERAZINO)-1,3-BENZOTHIAZOLE](/StructureFile/ChemBookStructure4/GIF/CB9399161.gif)
309285-78-7
7 suppliers
inquiry
Yield:309285-78-7 97%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 8 h;
Steps:
General synthesis of phenyl sulfonamide piperazine derivatives 9-21
General procedure: 2-(Piperazin-1-yl)benzazolyl derivatives 8a-d (1 equivalent) and triethylamine (1.5 equivalents) were dissolved in 25 ml dry CH2Cl2 and cooled to 0°C in an ice salt bath. Then the required phenylsulfonyl chloride (1.1 equivalents) dissolved in 20 ml dry CH2Cl2 was added dropwise. After the addition, the reaction mixture was stirred at 0°C for a further 1h and then allowed to warm to room temperature and the course of the reaction followed by TLC. After completion, the mixture was diluted with additional 50 ml of CH2Cl2 and extracted with 10% NaHCO3 solution (3 x 50 ml). The organic phase was washed with dist. H2O, sat. NaCl solution, dried over anhyd. Na2SO4, filtered and evaporated to dryness. The product was recrystallised from the appropriate solvent, the precipitate formed was filtered under suction and dried at 60°C under high vacuum.
References:
Hofer, Sandra;Kratschmar, Denise V.;Schernthanner, Brigitte;Vuorinen, Anna;Schuster, Daniela;Odermatt, Alex;Easmon, Johnny [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 19,p. 5397 - 5400] Location in patent:supporting information
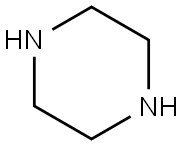
110-85-0
553 suppliers
$5.00/5G
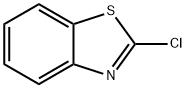
615-20-3
239 suppliers
$19.00/25g

98-59-9
588 suppliers
$9.00/5g
![2-(4-[(4-METHYLPHENYL)SULFONYL]PIPERAZINO)-1,3-BENZOTHIAZOLE](/StructureFile/ChemBookStructure4/GIF/CB9399161.gif)
309285-78-7
7 suppliers
inquiry

615-20-3
239 suppliers
$19.00/25g
![2-(4-[(4-METHYLPHENYL)SULFONYL]PIPERAZINO)-1,3-BENZOTHIAZOLE](/StructureFile/ChemBookStructure4/GIF/CB9399161.gif)
309285-78-7
7 suppliers
inquiry
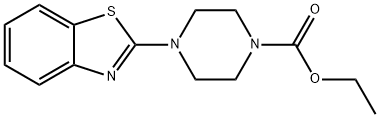
232263-41-1
0 suppliers
inquiry
![2-(4-[(4-METHYLPHENYL)SULFONYL]PIPERAZINO)-1,3-BENZOTHIAZOLE](/StructureFile/ChemBookStructure4/GIF/CB9399161.gif)
309285-78-7
7 suppliers
inquiry