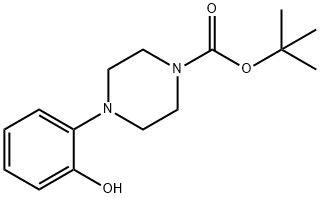
1-(2-HYDROXY-PHENYL)-PIPERAZINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:1-(2-HYDROXY-PHENYL)-PIPERAZINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:313657-51-1
- Molecular formula:C15H22N2O3
- Molecular Weight:278.35
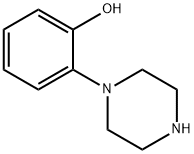
1011-17-2
148 suppliers
$45.00/25mg

24424-99-5
832 suppliers
$13.50/25G

313657-51-1
34 suppliers
$50.00/1g
Yield:313657-51-1 92%
Reaction Conditions:
in tetrahydrofuran at 60; for 6 h;
Steps:
12
Example 12; tert-Butyl 4-(2-hydroxyphenyl)piperazine-1-carboxylate (12) (Scheme 2); To a stirred suspension of 1-(2-hydroxyphenyl)piperazine (10) (1.78 g, 0.01 mol) in 20 mL tetrahydrofuran (in a round bottom flask fitted with a gas bubbler) was added a solution of di-tert-butylcarbonate 11 (2.62 g, 0.012 mol) in 10 mL tetrahydrofuran at room temperature. Soon it became a clear solution and a gas was evolved. The resulting mixture was heated at 60° C. for 6 h. The progress of the reaction was monitored by thin layer chromatography (TLC). The solvent was evaporated, the residue was dissolved in ethyl acetate (100 mL) and washed with water (100 mL×2), dried over sodium sulphate (Na2SO4) and evaporated. The residue was triturated with MTBE and filtered to give the compound 12. White solid, 2.5 g (92%). 1H NMR (400 MHz, CDCl3): δ 1.49 (s, 9H); 2.81(t, J=4.8 Hz, 4H); 3.58 (t, J=4.8 Hz, 4H); 6.84-6.88 (m, 1H); 6.94-6.99 (m, 1H); 7.06-7.12 (m, 2H). MS (ESI): m/z=279.50 (M+H+).
References:
US2008/293736,2008,A1 Location in patent:Page/Page column 25

24424-99-5
832 suppliers
$13.50/25G
58260-69-8
31 suppliers
$18.00/1g

313657-51-1
34 suppliers
$50.00/1g

95-56-7
305 suppliers
$9.00/5G

313657-51-1
34 suppliers
$50.00/1g
