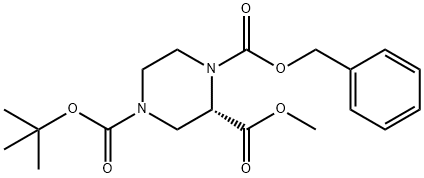
1,2,4-Piperazinetricarboxylic acid,4-(1,1-dimethylethyl)2-methyl1-(phenylmethyl)ester,(2S)- synthesis
- Product Name:1,2,4-Piperazinetricarboxylic acid,4-(1,1-dimethylethyl)2-methyl1-(phenylmethyl)ester,(2S)-
- CAS Number:314741-38-3
- Molecular formula:C19H26N2O6
- Molecular Weight:378.42

501-53-1
392 suppliers
$10.00/1g
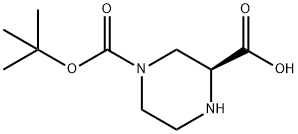
848482-93-9
124 suppliers
$9.00/100mg

74-88-4
341 suppliers
$15.00/10g

314741-38-3
5 suppliers
$2359.00/5g
Yield:314741-38-3 100%
Reaction Conditions:
Stage #1: (S)-4-(tert-butoxycarbonyl)piperazine-2-carboxylic acidwith sodium hydrogencarbonate in water at 20; for 0.5 h;
Stage #2: benzyl chloroformate in 1,4-dioxane;water at 20;
Stage #3: methyl iodidewith potassium carbonate in N,N-dimethyl-formamide at 20; for 2 h;
Steps:
Intermediate 226-i: I -benzyl 4-(tert-butyl) 2-methyl (S)-pi perazine-I ,2,4- tricarboxylate
To a stirred suspension of (S)-4-/V-Boc-piperazine-2-carboxylic acid (502 mg, 2.18 mmol) in water (2.5 mL) was added NaHC03(366.2 mg, 4.36 mmol), and the resulting suspension was stirred at ambient temperature for 30 minutes. A solution of benzyl chloroformate (744 mg, 4.36 mmol) in dioxane (4 mL) was then added and the reaction mixture was stirred at ambient temperature overnight. The reaction mixture was then diluted with water (5 mL) and extracted with EtOAc (2 15 mL). The combined organic layer was washed brine, dried over anhydrous Na2S04, filtered and concentrated under reduced pressure. The remaining residue was then dissolved in DMF (7 mL) and K2CO3 (904 mg, 6.54 mmol) was added. After stirring for 5 minutes, CH3I (928 mg, 6.54 mmol) was added slowly and the resulting mixture was stirred at ambient temperature for 2 h. The reaction was quenched with H2O and extracted with EtOAc (2X 15 mL).The combined organic layer was washed with brine, dried over anhydrous Na2S04, filtered and concentrated under reduced pressure to leave brown oil as the product (800 mg, quant.).1H NMR (400 MHz, CDCI3) δ 7.42 - 7.31 (m, 5H), 5.22 - 5.09 (m, 2H), 4.84 - 4.63 (m, 1 H), 4.63 - 4.47 (m, 1 H), 4.11 - 3.81 (m, 2H), 3.72 (d, J = 22.6 Hz, 3H), 3.32 (s, H), 3.08 (d, J = 13.5 Hz, H), .44 (s, 9H). Mass calculated for (Ci9H26N206+H)+379.2, found 379.1.
References:
WO2017/75694,2017,A1 Location in patent:Page/Page column 327; 328
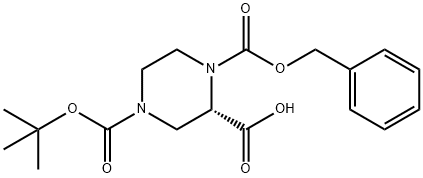
150407-69-5
71 suppliers
$60.00/100mg
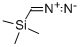
18107-18-1
207 suppliers
$33.00/5g

314741-38-3
5 suppliers
$2359.00/5g

150407-69-5
71 suppliers
$60.00/100mg

74-88-4
341 suppliers
$15.00/10g

314741-38-3
5 suppliers
$2359.00/5g

848482-93-9
124 suppliers
$9.00/100mg

314741-38-3
5 suppliers
$2359.00/5g

501-53-1
392 suppliers
$10.00/1g

314741-38-3
5 suppliers
$2359.00/5g