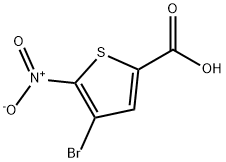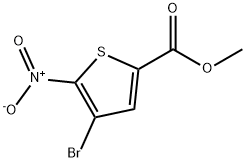
2-Thiophenecarboxylic acid, 4-bromo-5-nitro-, methyl ester synthesis
- Product Name:2-Thiophenecarboxylic acid, 4-bromo-5-nitro-, methyl ester
- CAS Number:31862-80-3
- Molecular formula:C6H4BrNO4S
- Molecular Weight:266.07
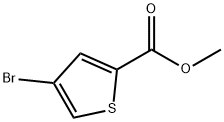
62224-16-2
125 suppliers
$13.00/1g

31862-80-3
16 suppliers
inquiry
Yield:31862-80-3 99.3%
Reaction Conditions:
with sulfuric acid;HNO3 in dichloromethane; for 0.5 h;Cooling with ice;
Steps:
1.1 methyl 4-bromo-5-nitrothiophene-2-carboxylate
Under ice-salt bath conditions, 1a (2.2 g, 10.0 mmol) was added to concentrated sulfuric acid (10 mL), and fuming nitric acid (945.0 mg, 15.0 mmol) was dissolved in concentrated sulfuric acid (5 mL) and slowly added dropwise to the reaction solution After the dropwise addition, the reaction was continued under an ice-salt bath for 30 minutes. The reaction solution was slowly poured into the ice-water solution, and a large amount of white solid was precipitated. The filter cake was collected by filtration, washed with water (10 mL×2), and dried to obtain 1b (2.6 g, yield 99.3%).
References:
WO2022/135572,2022,A1 Location in patent:Page/Page column 37-38