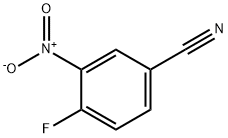
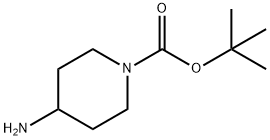
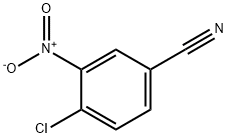
TERT-BUTYL 4-(4-CYANO-2-NITROPHENYLAMINO)PIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-(4-CYANO-2-NITROPHENYLAMINO)PIPERIDINE-1-CARBOXYLATE
- CAS Number:320406-05-1
- Molecular formula:C17H22N4O4
- Molecular Weight:346.38
Yield:320406-05-1 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20; for 72 h;
Steps:
Preparation of tert-butyl 4-[(4-cyano-2-nitrophenyl)amino]piperidine-1- carboxylate; tert-butyl 4-[(4-cyano-2-nitrophenyl)amino]piperidine-1-carboxylate
References:
WO2008/84300,2008,A1 Location in patent:Page/Page column 84-85