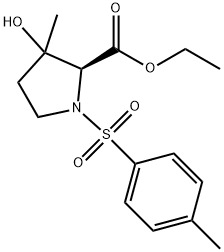
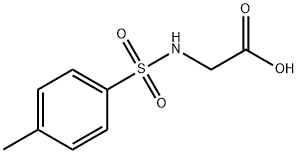
ethyl 3-hydroxy-3-methyl-1-tosylpyrrolidine-2-carboxylate synthesis
- Product Name:ethyl 3-hydroxy-3-methyl-1-tosylpyrrolidine-2-carboxylate
- CAS Number:3284-52-4
- Molecular formula:C15H21NO5S
- Molecular Weight:327.4
Yield:3284-52-4 76%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in tetrahydrofuran at 20;
Steps:
32 Synthesis of 3-Hydroxy-3-methyl-1-(toluene-4-sulfonyl)-pyrrolidine-2-carboxylic acid ethyl ester (82)
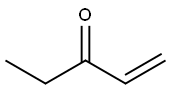
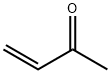
1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) (7 mL, 47.08 mmol) was added to a stirred solution of ethyl vinyl ketone (1.75 mL, 21.4 mmol) and ethyl N-p-toluenesulfonylglycinate 81 (5.5 g, 21.4 mL) in THF (50 mL). The resulting mixture was stirred overnight at room temperature. The mixture was diluted with ether, washed with 5% aq. HCl, 5% sodium bicarbonate solution and water. The combined organics were dried with Na2SO4, filtered, evaporated under reduced pressure to give crude 82 (5.3 g, 76%) as a yellow oil (diastereoisomers mixture). 1H-NMR (400 MHz, CDCl3): δ 1.29 (m, 6H), 1.75 (m, 1H), 2.09 (m, 1H), 2.43 (s, 3H), 3.40 (m, 1H), 3.56 (m, 1H), 4.04 (s, 1H), 4.20 (m, 2H), 7.30 (d, 2H), 7.75 (d, 2H) ppm. 13C-NMR (100 MHz, CDCl3): δ 14.12, 15.28, 23.04, 25.60, 26.27, 38.20, 38.86, 46.26, 46.46, 61.49, 61.65, 69.10, 71.69, 127.51, 129.70, 134.83, 134.91, 143.58, 143.84, 170.03, 170.45 ppm. DEPT (100 MHz, CDCl3): CH3 carbons: 14.12, 15.28, 23.04, 25.60, 26.27; CH2 carbons: 38.20, 38.86, 46.26, 46.46, 61.49, 61.65; CH carbons: 69.10, 71.69, 127.51, 129.70 ppm. LC/MS: 97.60%, m/z=327.
References:
US2005/143443,2005,A1 Location in patent:Page/Page column 39-40
3299-38-5
52 suppliers
$45.00/100mg

5465-67-8
47 suppliers
$28.60/50MG

3284-52-4
21 suppliers
inquiry

98-59-9
601 suppliers
$9.00/5g

3284-52-4
21 suppliers
inquiry
1080-44-0
168 suppliers
$10.00/1g

3284-52-4
21 suppliers
inquiry