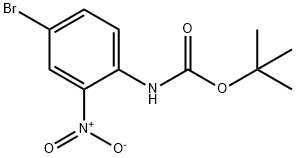
N-(3-Nitro[1,1'-biphenyl]-4-yl)carbaMic Acid tert-Butyl Ester synthesis
- Product Name:N-(3-Nitro[1,1'-biphenyl]-4-yl)carbaMic Acid tert-Butyl Ester
- CAS Number:335254-77-8
- Molecular formula:C17H18N2O4
- Molecular Weight:314.34
Yield:335254-77-8 73%
Reaction Conditions:
with tetrakis-(triphenylphosphine)-palladium;potassium carbonate in tetrahydrofuran; for 15 h;Reflux;Suzuki Coupling;
Steps:
tert-Butyl 3-nitrobiphenyl-4-ylcarbamate (3b).
Compound 2 (0.32 g, 1.00 mmol), benzene-2-boronic acid (0.15 g, 1.20 mmol), Pd(PPh3)4 (0.06 g, 0.05 mmol), K2CO3 (0.55 g, 4.00 mmol) were added to THF. The reaction mixture was stirred at reflux for 15h before evaporation to dryness. The crude solid was extracted with ethyl acetate (50 mL) and washed with water (50 mL x 2). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. Purification using flash column chromatography (dichloromethane - hexane 1:4 to 1:3) on silica gel yielded compound 3b (0.23 g, 73%) as yellow solid. m.p. 125 - 127 °C. 1H NMR (400 MHz, CDCl3) δ 9.69 (s, 1H), 8.65 (d, J = 8.8 Hz, 1H), 8.44 (s, 1H), 7.87 (d, J = 8.4 Hz, 1H), 7.61 (d, J = 7.2 Hz, 2H), 7.49 (t, J = 7.6 Hz, 2H), 7.41 (t, J = 7.2 Hz, 1H), 1.59 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 152.2, 138.2, 136.2, 135.1, 134.9, 134.1, 129.1, 128.1, 126.7, 123.7, 121.2, 81.9, 28.2. HRMS (ESI) m/z (M+H)+ calcd for C17H19N2O4 = 315.1345; found 315.1348.
References:
La, Minh Thanh;Jeong, Byung-Hoon;Kim, Hee-Kwon [Bulletin of the Korean Chemical Society,2021,vol. 42,# 5,p. 740 - 743] Location in patent:supporting information
875-51-4
302 suppliers
$5.00/1g
![N-(3-Nitro[1,1'-biphenyl]-4-yl)carbaMic Acid tert-Butyl Ester](/CAS/GIF/335254-77-8.gif)
335254-77-8
12 suppliers
inquiry

24424-99-5
823 suppliers
$13.50/25G
![N-(3-Nitro[1,1'-biphenyl]-4-yl)carbaMic Acid tert-Butyl Ester](/CAS/GIF/335254-77-8.gif)
335254-77-8
12 suppliers
inquiry

335254-69-8
17 suppliers
$129.00/100mg

98-80-6
719 suppliers
$5.00/5g
![N-(3-Nitro[1,1'-biphenyl]-4-yl)carbaMic Acid tert-Butyl Ester](/CAS/GIF/335254-77-8.gif)
335254-77-8
12 suppliers
inquiry