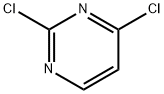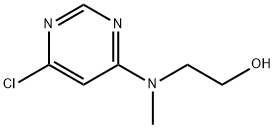
2-[(6-Chloro-4-pyrimidinyl)(methyl)amino]-1-ethanol synthesis
- Product Name:2-[(6-Chloro-4-pyrimidinyl)(methyl)amino]-1-ethanol
- CAS Number:340742-83-8
- Molecular formula:C7H10ClN3O
- Molecular Weight:187.63

109-83-1
431 suppliers
$10.00/25ml
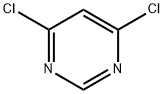
1193-21-1
555 suppliers
$14.00/5g
![2-[(6-Chloro-4-pyrimidinyl)(methyl)amino]-1-ethanol](/CAS/GIF/340742-83-8.gif)
340742-83-8
9 suppliers
inquiry
Yield:340742-83-8 93%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in ethanol at 0;Microwave irradiation;Reagent/catalyst;Temperature;
Steps:
6 Synthesis of Compound 82c and Compound 82d by Method B
In separate experiments, compounds 82c and 82d were obtained by reacting 4,6-dichloropyrimidine (1.0 mmol,0.148 g) with the appropriate amine (2-(methylamino)etha- nol for compound 82c and morpholine for compound 82d;1.0 mmol) in ethanol (2-3 ml) at 0° C. in the presence ofN,N-diisopropylethylamine (DIPEA, 1.1 mmol) under microwave irradiation for 10-20 mm. The progress of reaction was monitored by TLC. Ethyl acetate (10.0 ml) was added to the reaction mixture, and the pH was adjusted to7-7.5 using RC1 (6.0 M). The mixture was washed withsaturated aqueous solution of NaRCO3. The organic layer was dried over anhydrous Mg504. The excess solvent wasremoved under reduced pressure, and the obtained residue was further purified by column chromatography using ethylacetate-hexane (1:1) to afford the final products 82c and 82d,respectively in good yields. See Table 1.
References:
US10702525,2020,B1 Location in patent:Page/Page column 6