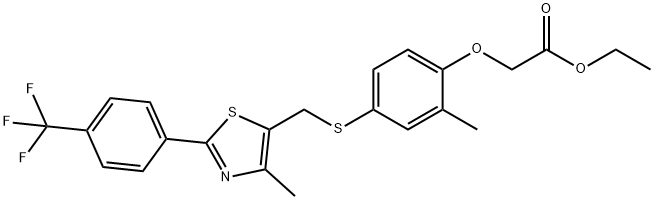
2-[2-Methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]acetic acid ethyl ester synthesis
- Product Name:2-[2-Methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]acetic acid ethyl ester
- CAS Number:343322-83-8
- Molecular formula:C23H22F3NO3S2
- Molecular Weight:481.55
Yield:343322-83-8 98.5%
Reaction Conditions:
with potassium carbonate in acetone at -10; for 4 h;
Steps:
15 Example 15; Preparation of ethyl {2-methyl-4-[4-methyl-2-(4-trifluoromethyl-phenyl)- thiazole-5-yl sulfanyl] phenoxy} acetate [Step G]:
2-Methyl-4- [4-METHYL-2- [ (4-TRIFLUOROMETHYL)] phenyl] [THIAZOLE-5-YL] methyl- sulfanyl} phenol (10.0 g, [25. 3 MMOL)] obtained from Example [13] was dissolved in acetone [(350 MNo.), ] and potassium carbonate (8.0 g, [58.] 2 mmol. [2. 3] eq. ) was added at room temperature. Ethyl bromoacetate (4.2 [TLE,] [38.] 0 mmol, [1.] [5] eq. ) was added for 4 hours while stirring vigorously. After completion of reaction, the mixture was extracted with brine and ethyl acetate, and the organic layer was dried over magnesium sulfate. The solvent was evaporated under reduced pressure, and the residue was purified on silica flash column to thereby yield 11.8 [G] of the title compound (yield: 98. 5%).
References:
WO2003/106442,2003,A1 Location in patent:Page 18-19
![Thiazole,5-(chloromethyl)-4-methyl-2-[4-(trifluoromethyl)phenyl]-](/CAS/GIF/317318-97-1.gif)
317318-97-1
85 suppliers
$11.00/1g
![2-[2-Methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]acetic acid ethyl ester](/CAS2/GIF/343322-83-8.gif)
343322-83-8
7 suppliers
inquiry

2362-12-1
187 suppliers
$5.00/1g
![2-[2-Methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]acetic acid ethyl ester](/CAS2/GIF/343322-83-8.gif)
343322-83-8
7 suppliers
inquiry
72505-21-6
158 suppliers
$10.00/5g
![2-[2-Methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]acetic acid ethyl ester](/CAS2/GIF/343322-83-8.gif)
343322-83-8
7 suppliers
inquiry
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
67 suppliers
$65.00/50mg
![2-[2-Methyl-4-[[[4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl]methyl]thio]phenoxy]acetic acid ethyl ester](/CAS2/GIF/343322-83-8.gif)
343322-83-8
7 suppliers
inquiry

