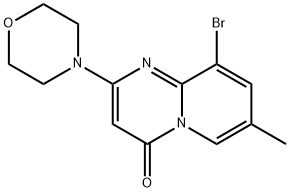
4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-7-methyl-2-(4-morpholinyl)- synthesis
- Product Name:4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-7-methyl-2-(4-morpholinyl)-
- CAS Number:351002-16-9
- Molecular formula:C13H14BrN3O2
- Molecular Weight:324.17

110-91-8
677 suppliers
$9.00/1g
1173900-34-9
0 suppliers
inquiry
![4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-7-methyl-2-(4-morpholinyl)-](/CAS/GIF/351002-16-9.gif)
351002-16-9
48 suppliers
$165.00/100mg
Yield:351002-16-9 94%
Reaction Conditions:
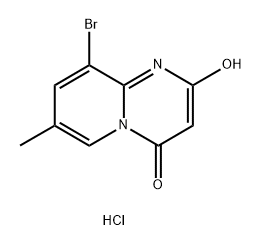
Stage #1: 9-bromo-2-hydroxy-7-methyl-4H-pyrido[1,2-a]pyrimidin-4-one hydrochloridewith triethylamine in tetrahydrofuran at 5; for 0.166667 h;
Stage #2: with methanesulfonyl chloride in tetrahydrofuran at 5;
Stage #3: morpholine in tetrahydrofuran;water at 20 - 65;
Steps:
1.a
Example 1; (-) 2-[1-(7-Methyl-2-(morpholin-4-yl)-4-oxo-4H-pyrido[1,2-a]pyrimidin-9-yl)ethylamino]benzoic acid, probably (-) 2-{[(1R)-1-(7-methyl-2-morpholin-4-yl-4-oxo-4H-pyrido[1,2-a]pyrimidin-9-yl)ethyl]amino}benzoic acid; a) 9-Bromo-7-methyl-2-morpholin-4-yl-4H-pyrido[1,2-a]pyrimidin-4-one; To a stirred slurry of 9-bromo-2-hydroxy-7-methyl-4H-pyrido[1,2-a]pyrimidin-4-one hydrochloride (407 g, 1.40 mol) in dry THF (4 L) at 5° C. under a N2-atm, triethylamine (259 g, 2.56 mol) was added over 10 min. MsCl (259 g, 2.26 mol) was added over 30 min and the resulting mixture stirred for 2.5 h at 5° C. Morpholine (388 g, 4.45 mol) was added and the resulting mixture stirred at 60° C. for 6 h. Water (8.2 L) was added and the resulting mixture stirred at 65° C. for 3 h. The mixture was cooled to 20° C. during 4 h and stirred at 20° C. over night. The product was filtered off, washed with water (2.0 L+1.5 L) and dried under vacuum at 0-1 mbar and 40° C. to yield 426 g (94%) of the subtitle compound.1H NMR (400 MHz, CDC3) δ 8.71 (s, 1H), 7.84 (s, 1H), 5.60 (s, 1H), 3.89-3.60 (m, 8H), 2.34 (s, 3H).
References:
US2009/191177,2009,A1 Location in patent:Page/Page column 7

110-91-8
677 suppliers
$9.00/1g
![4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-2-hydroxy-7-methyl-](/CAS/GIF/663619-90-7.gif)
663619-90-7
46 suppliers
$68.00/100mg
![4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-7-methyl-2-(4-morpholinyl)-](/CAS/GIF/351002-16-9.gif)
351002-16-9
48 suppliers
$165.00/100mg
17282-00-7
322 suppliers
$5.00/1g
![4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-7-methyl-2-(4-morpholinyl)-](/CAS/GIF/351002-16-9.gif)
351002-16-9
48 suppliers
$165.00/100mg
![4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-2-hydroxy-7-methyl-](/CAS/GIF/663619-90-7.gif)
663619-90-7
46 suppliers
$68.00/100mg
![4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-7-methyl-2-(4-morpholinyl)-](/CAS/GIF/351002-16-9.gif)
351002-16-9
48 suppliers
$165.00/100mg
1603-41-4
537 suppliers
$7.00/25g
![4H-Pyrido[1,2-a]pyrimidin-4-one, 9-bromo-7-methyl-2-(4-morpholinyl)-](/CAS/GIF/351002-16-9.gif)
351002-16-9
48 suppliers
$165.00/100mg