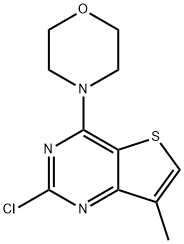
Thieno[3,2-d]pyriMidine, 2-chloro-7-Methyl-4-(4-Morpholinyl)- synthesis
- Product Name:Thieno[3,2-d]pyriMidine, 2-chloro-7-Methyl-4-(4-Morpholinyl)-
- CAS Number:35265-88-4
- Molecular formula:C11H12ClN3OS
- Molecular Weight:269.75

110-91-8
678 suppliers
$9.00/1g
![2,4-Dichloro-7-methylthieno[3,2-d]pyrimidine](/CAS/GIF/35265-83-9.gif)
35265-83-9
89 suppliers
$72.50/250mg
![Thieno[3,2-d]pyriMidine, 2-chloro-7-Methyl-4-(4-Morpholinyl)-](/CAS/20150408/GIF/35265-88-4.gif)
35265-88-4
31 suppliers
$456.00/1g
Yield:35265-88-4 95%
Reaction Conditions:
in methanol;
Steps:
3 4-(2-Chloro-7-methylthieno[3,2-d]pyrimidin-4-yl)morpholino VI
Example 3
4-(2-Chloro-7-methylthieno[3,2-d]pyrimidin-4-yl)morpholino VI
A mixture of 2,4-dichloro-7-methylthieno[3,2-d]pyrimidine VII (90 g, 0.411 mol) and methanol (900 mL) was cooled to 10° C. Morpholine (89.5 g, 1.03 mol) was added while maintaining the temperature below 15° C.
The reaction mixture was stirred for 2 h and then cooled to 5° C. and stirred for additional 1 h.
The solid was collected by filtration, washed with water (450 mL) and dried under reduced pressure at 50° C. for 24 h to afford 4-(2-chloro-7-methylthieno[3,2-d]pyrimidin-4-yl)morpholino VI as a white solid (105 g, 95% yield).
1H NMR (400 MHz, CDCl3) δ 7.94 (s, 1H), 3.95-3.86 (m, 4H), 3.80-3.71 (m, 4H), 2.9 (s, 3H); LCMS (ESI pos) m/z [M+H] 270
References:
US2014/100366,2014,A1 Location in patent:Page/Page column
85006-31-1
357 suppliers
$5.00/5g
![Thieno[3,2-d]pyriMidine, 2-chloro-7-Methyl-4-(4-Morpholinyl)-](/CAS/20150408/GIF/35265-88-4.gif)
35265-88-4
31 suppliers
$456.00/1g
![7-Methylthieno[3,2-d]pyrimidine-2,4-diol](/CAS/GIF/35265-81-7.gif)
35265-81-7
38 suppliers
$45.00/10mg
![Thieno[3,2-d]pyriMidine, 2-chloro-7-Methyl-4-(4-Morpholinyl)-](/CAS/20150408/GIF/35265-88-4.gif)
35265-88-4
31 suppliers
$456.00/1g