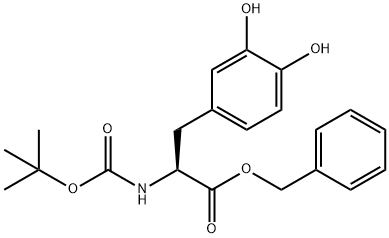
N-(tert-butoxycarbonyl)-3,4-dihydroxy-L-Pheny lalanine benzyl ester synthesis
- Product Name:N-(tert-butoxycarbonyl)-3,4-dihydroxy-L-Pheny lalanine benzyl ester
- CAS Number:37169-37-2
- Molecular formula:C21H25NO6
- Molecular Weight:387.43

100-39-0
424 suppliers
$10.00/10 g
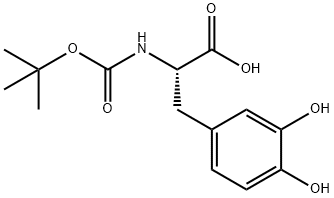
30033-24-0
49 suppliers
$146.66/0.5g

37169-37-2
18 suppliers
$275.00/1g
Yield: 16.2 g
Reaction Conditions:
with potassium hydrogencarbonate in N,N-dimethyl-formamide at 20; for 7 h;Inert atmosphere;
Steps:
2
The crude product obtained in the previous step was dissolved in N,N-dimethylformamide (51 mL). To this solution, potassium hydrogen carbonate (7.6 g) was added under an argon atmosphere, and then, benzyl bromide (7.3 mL) was added thereto. The resulting solution was stirred at room temperature for 7 hours. To the reaction mixture, 2 N hydrochloric acid (92 mL) was added under ice-cooling to acidify the solution, and then, extraction was performed with a mixed solution of n-heptane and ethyl acetate (1:1) (150 mL×2). The organic layers were combined and washed with water (75 mL×2) and a saturated aqueous solution of sodium chloride (75 mL), and then dried over magnesium sulfate. After magnesium sulfate was removed by filtration, the solvent was concentrated under reduced pressure. The resulting residue was recrystallized from ethyl acetate/n-heptane, whereby the title compound (16.2 g, 2-step yield: 82%) having the following physical properties was obtained. [0357] TLC (Rf value): 0.64 (n-hexane:ethyl acetate:acetic acid=50:50:1) [0358] NMR (300 MHz, CDCl3): δ 7.31-7.40 (m, 5H), 6.98 (d, J=7.8 Hz, 1H), 6.44 (dd, J=7.8, 1.8 Hz, 1H), 6.40 (d, J=1.8 Hz, 1H), 5.26-5.64 (br, 2H), 5.05-5.23 (m, 2H), 5.00 (d, J=8.1 Hz, 1H), 4.50-4.58 (m, 1H), 2.94 (d, J=5.7 Hz, 2H), 1.41 (s, 9H)
References:
ONO PHARMACEUTICALS CO., LTD.;Kokubo, Masaya;Yano, Koji US2013/245074, 2013, A1 Location in patent:Paragraph 0356-0358

24424-99-5
824 suppliers
$13.50/25G
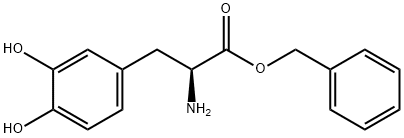
55720-47-3
0 suppliers
inquiry

37169-37-2
18 suppliers
$275.00/1g
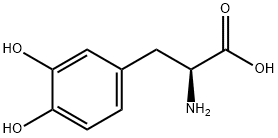
59-92-7
657 suppliers
$14.00/5g

37169-37-2
18 suppliers
$275.00/1g

24424-99-5
824 suppliers
$13.50/25G

37169-37-2
18 suppliers
$275.00/1g