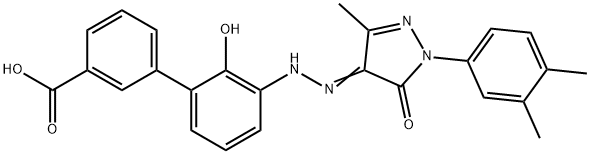
3'-[[1-(3,4-DiMethylphenyl)-1,5-dihydro-3-Methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid synthesis
- Product Name:3'-[[1-(3,4-DiMethylphenyl)-1,5-dihydro-3-Methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid
- CAS Number:376591-99-0
- Molecular formula:C25H22N4O4
- Molecular Weight:442.47
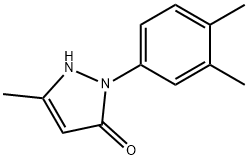
277299-70-4
221 suppliers
$20.00/1g
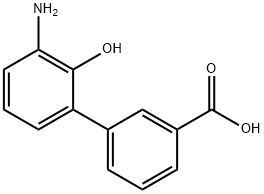
![3'-aMino-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid hydrochloride](/CAS/GIF/376591-97-8.gif)
376591-97-8
36 suppliers
$495.75/5MG
![3'-[[1-(3,4-DiMethylphenyl)-1,5-dihydro-3-Methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid](/CAS/20150408/GIF/376591-99-0.gif)
376591-99-0
10 suppliers
inquiry
Yield:376591-99-0 90%
Reaction Conditions:
Stage #1: 3'-amino-2'-hydroxy-1,1'-biphenyl-3-carboxylic acid hydrochloridewith hydrogenchloride;sodium nitrite in methanol;water; pH=1-2; for 2.5 h;
Stage #2: 2-(3,4-dimethylphenyl)-1,2-dihydro-5-methyl-3H-pyrazol-3-onewith sodium hydrogencarbonate in methanol;water at 20; pH=8; for 12 h;
Stage #3: with hydrogenchloride in methanol;water at 0 - 20; pH=1-2; for 3 h;
Steps:
1.3; 2.3; 3.3; 4.3; 5.3; 6.3; 7.3; 8.3; 9.3; 10.3; 11.3; 12.3; 13.3; 14.3; 15.3; 16.3; 17.3
(3) Add 3.36mL hydrochloric acid (4mol/L) and 20mL methanol into the first reaction chamber,Dissolve the solid intermediate AQ-2 as material II (pH value 1-2),Open the material valve between the first reaction chamber and the second reaction chamber,The first reaction chamber is connected to a gas pump to press the material II into the second reaction chamber,Then slowly add an aqueous solution of sodium nitrite (0.407g of sodium nitrite dissolved in 2mL of water to make a solution), the dropwise addition is completed within 30 minutes, and the reaction is continuously stirred for 2 hours during the dropping process.After the reaction, adjust the pH to 8 with sodium bicarbonate solution;Take 1.08g of 2-(3,4-dimethylphenyl)-1,2-dihydro-5-methyl-3H-pyrazol-3-one and dissolve it in 10mL of methanol,It is delivered to the second reaction chamber by a peristaltic pump,After reacting at 20°C for 12 hours, it is cooled to 0-5°C, the pH of the reaction solution is adjusted to 1-2 with 4moL/L hydrochloric acid, and the reaction is continued to stir at room temperature for 3 hours, followed by distillation and washing under reduced pressure.Recrystallize with a mixed solution of tetrahydrofuran and water,After drying, 1.43g of khaki solid intermediate AQ-4 was obtained, with a yield of 90%;
References:
CN111518029,2020,A Location in patent:Paragraph 0036; 0039; 0041; 0044; 0046; 0049; 0051; 0054;
376592-93-7
257 suppliers
$18.00/100mg
18048-64-1
146 suppliers
$6.00/250mg
![3'-[[1-(3,4-DiMethylphenyl)-1,5-dihydro-3-Methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid](/CAS/20150408/GIF/376591-99-0.gif)
376591-99-0
10 suppliers
inquiry
116557-46-1
155 suppliers
$9.00/250mg
![3'-[[1-(3,4-DiMethylphenyl)-1,5-dihydro-3-Methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid](/CAS/20150408/GIF/376591-99-0.gif)
376591-99-0
10 suppliers
inquiry
![[1,1'-Biphenyl]-3-carboxylic acid, 3'-amino-2'-methoxy-](/CAS/20210111/GIF/1176777-76-6.gif)
1176777-76-6
8 suppliers
inquiry
![3'-[[1-(3,4-DiMethylphenyl)-1,5-dihydro-3-Methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid](/CAS/20150408/GIF/376591-99-0.gif)
376591-99-0
10 suppliers
inquiry
25487-66-5
462 suppliers
$6.00/5g
![3'-[[1-(3,4-DiMethylphenyl)-1,5-dihydro-3-Methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazino]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid](/CAS/20150408/GIF/376591-99-0.gif)
376591-99-0
10 suppliers
inquiry