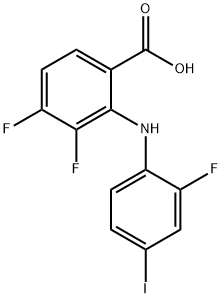
2-(2-Fluoro-4-iodoanilino)-3,4-difluorobenzoic Acid synthesis
- Product Name:2-(2-Fluoro-4-iodoanilino)-3,4-difluorobenzoic Acid
- CAS Number:391211-97-5
- Molecular formula:C13H7F3INO2
- Molecular Weight:393.1
29632-74-4
380 suppliers
$6.00/5g

61079-72-9
366 suppliers
$6.00/1g

391211-97-5
103 suppliers
$62.00/100mg
Yield:391211-97-5 99%
Reaction Conditions:
with lithium amide in tetrahydrofuran;
Steps:
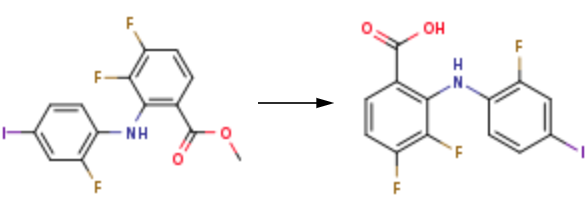
2.11. Synthesis of A 2A R PAM-1
A solution of 2,3,4-uorobenzoic acid (1.35 g, 7.68 mmol), 2-uoro-4-iodoaniline (1.91 g, 8.06 mmol), and lithium amide (0.702 g,30.6 mmol) in tetrahydrofuran (10.5 ml) was reacted using a standardmethod (Cai et al., 2008) to give 3,4-diuoro-2-((2-uoro-4-iodo-phenyl)amino)benzoic acid (A 2A R PAM-1, 2.99 g, 99%) as a brown solid(Figure S1); IR (KBr) 3311, 1673, 1602, 1520, 1500, 1444, 1273,768 cm-1;1H NMR (400 MHz CD 3 OD) δ = 7.89 (1 H, ddd, J = 2.3, 6.0,9.2 Hz), 7.48 (1 H, dd, J = 1.8, 10.5 Hz), 7.41 (1 H, ddd, J = 1.4, 1.8,8.5 Hz), 6.91 (1 H, ddd, J = 7.3, 9.4, 9.4 Hz), 6.75 (1 H, ddd, J = 5.6,8.5, 8.5 Hz);13C NMR (100 MHz acetone-d 6 ) δ = 169.9, 155.7 (dd,J C,F = 252.1, 4.8 Hz), 155.6 (d, J C,F = 252.1 Hz), 143.6 (dd,J C,F = 247.8, 14.9 Hz), 137.4 (dd, J C,F = 7.7, 2.9 Hz), 135.0 (d,J C,F = 3.8 Hz), 131.9(d, J C,F = 11.5 Hz), 129.8 (dd, J C,F = 9.6, 3.8 Hz),125.8 (d, J C,F = 21.0 Hz), 123.8 (d, J C,F = 5.8 Hz), 116.4, 110.1 (d,J C,F = 18.2 Hz), 84.7 (d, J C,F = 6.7 Hz); HRMS-ESI: m/z [M-H]-calcdfor C13H6F3INO2, 391.9395; measured, 391.9414.
References:
Korkutata, Mustafa;Saitoh, Tsuyoshi;Cherasse, Yoan;Ioka, Shuji;Duo, Feng;Qin, Rujie;Murakoshi, Nobuyuki;Fujii, Shinya;Zhou, Xuzhao;Sugiyama, Fumihiro;Chen, Jiang-Fan;Kumagai, Hidetoshi;Nagase, Hiroshi;Lazarus, Michael [Neuropharmacology,2019,vol. 144,p. 122 - 132]

29632-74-4
380 suppliers
$6.00/5g

391211-97-5
103 suppliers
$62.00/100mg