4-(1H-IMIDAZOL-4-YL)PHENYL METHYL ETHER synthesis
- Product Name:4-(1H-IMIDAZOL-4-YL)PHENYL METHYL ETHER
- CAS Number:35512-31-3
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
Yield:35512-31-3 87%
Reaction Conditions:
at 170; for 6 h;
Steps:
15.1 4-(4-Methoxyphenyl)-1H-imidazole
Reference Example 15-1 4-(4-Methoxyphenyl)-1H-imidazole 4-Methoxyphenacyl bromide (2.29 g, 10.0 mmol) was dissolved in formamide (45.0 g, 1.00 mol), and the mixture was stirred at 170°C for 6 hours. The reaction solution was cooled to room temperature, and thereto was added water. The mixture was extracted with ethyl acetate, and the organic layer was washed successively with water and saturated saline, and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, and thereto was added a mixture of hexane and ethyl acetate (5:1) (200 ml). The resulting suspension was stirred at 50°C for 2 hours, and further stirred at room temperature for 5 hours. The precipitated crystals were collected by filtration, and washed with hexane to give the title compound (1.52 g, 87 %). 1H NMR (CDCl3, 400 MHz) δ 8.05 (brs, 1H), 7.68 (d, 1H, J = 1.1Hz), 7.63 (d, 2H, J = 8.9 Hz), 7.23 (d, 1H, J = 1.1Hz), 6.91 (d, 2H, J = 8.9 Hz), 3.81 (s, 3H).
References:
EP1647546,2006,A1 Location in patent:Page/Page column 35

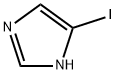
768-60-5
225 suppliers
$6.00/1g

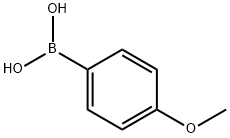
35512-31-3
14 suppliers
inquiry
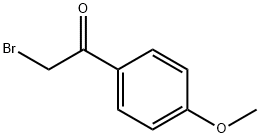
2632-13-5
367 suppliers
$5.00/25mg

35512-31-3
14 suppliers
inquiry