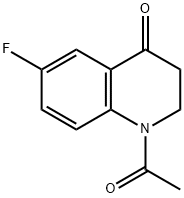
4(1H)-Quinolinone, 1-acetyl-6-fluoro-2,3-dihydro- synthesis
- Product Name:4(1H)-Quinolinone, 1-acetyl-6-fluoro-2,3-dihydro-
- CAS Number:38470-30-3
- Molecular formula:C11H10FNO2
- Molecular Weight:207.2
Yield:38470-30-3 0.55 g
Reaction Conditions:
with pyridine in dichloromethane at 0 - 20; for 3 h;
Steps:
General procedure: To a solution of aniline (4.3 mL, 4.40 g, 47.25 mmol) and NaHCO3 (4.37 g,51.98 mmol) in CH3CN (50 mL) at 0 °C was added dropwise3-bromopropanoyl chloride (2, 8.91 g, 51.98 mmol). The mixture was stirred at0 °C for a couple of minutes and allowed to warm to room temperature andkept at this temperature for another 3 h. Then the reaction was quenched with water and extracted with EtOAc (20 mL 3). The combined organic layerswere evaporated, and the residue was grinded with petroleum ether/EtOAc(5:1) to give 3-bromo-N-phenylpropanamide (3a, 8.41 g, 78%) as a brown solid [2]. This solid was then dissolved in DMF (10 mL) and added slowly into asolution of t-BuNa (3.90 g, 40.56 mmol) in DMF (70 mL). The resultant mixture was allowed to warm up to room temperature gradually. The reaction wasstirred at room temperature for 3 h, quenched with water and extracted with EtOAc (20 mL 3). The organic layers were collected, the volatiles evaporated under reduced pressure, and the residue was grinded with MTBE to give 1-phenylazetidin-2-one (4a, 3.53 g, 65%) as a red solid [2].To a solution of 1-phenylazetidin-2-one (4a, 3.53 g, 23.97 mmol) in DCE (40 mL) at 0 °C was added TfOH (4.67 mL, 52.77 mmol). The mixture was allowed to warm up to room temperature and stirred for 2 h. The reaction was quenched with aq. NaHCO3 and extracted with EtOAc (15 mL 3). The combined organic layers were separated, washed with brine, dried over Na2SO4, filtered, and concentrated to give the crude 2,3-dihydroquinolin-4(1H)-one (5a, 3.53 g, calculated as ~100%) as a yellow solid, which was used for next step directly without further purification [2]. The crude 2,3-dihydroquinolin-4(1H)-one (5a) was dissolved in CH2Cl2 (20 mL).Then, pyridine (2.68 g, 33.29 mmol) was added followed by the dropwise addition of acetyl chloride (2.24 g, 28.54 mmol) at 0 °C. After completion of the addition, the reaction was allowed to warm to room temperature and stirred for3 h. Then the solvent was removed under reduce pressure, and the residue was partitioned between EtOAc (30 mL) and brine. The organic layer was separated, dried over MgSO4, filtered, and concentrated. The crude product1-acetyl-2,3-dihydroquinolin-4(1H)-one (6a) was purified by flash column chromatography on silica gel with EtOAc/petroleum ether (boiling point range 60-90 °C) (v/v 1:3) as eluent to afford the pure compound 1-acetyl-2,3-dihydroquinolin-4(1H)-one (6a) as a light brown solid (4.18 g, 47% overall yield) [3].
References:
Luan, Lin-bo;Song, Zi-jie;Li, Zhi-ming;Wang, Quan-rui [Beilstein Journal of Organic Chemistry,2018,vol. 14,p. 1826 - 1833] Location in patent:supporting information
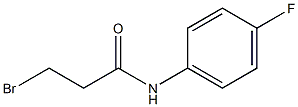
135154-75-5
3 suppliers
inquiry

38470-30-3
4 suppliers
inquiry
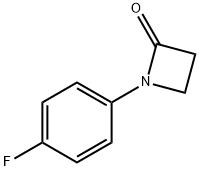
135154-76-6
3 suppliers
inquiry

38470-30-3
4 suppliers
inquiry
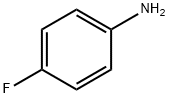
371-40-4
404 suppliers
$5.00/5G

38470-30-3
4 suppliers
inquiry

