
4-(2-CHLORO-6-METHYLPYRIMIDIN-4-YL)MORPHOLINE synthesis
- Product Name:4-(2-CHLORO-6-METHYLPYRIMIDIN-4-YL)MORPHOLINE
- CAS Number:52026-43-4
- Molecular formula:C9H12ClN3O
- Molecular Weight:213.66

110-91-8
695 suppliers
$9.00/1g
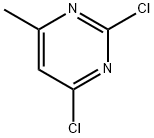
5424-21-5
330 suppliers
$10.00/5g

52026-43-4
23 suppliers
inquiry
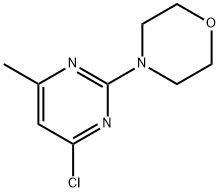
118121-82-7
16 suppliers
$49.00/200 μmol
Yield: 70% , 24%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 20;
Steps:
96.a
a) 4-(2-Chloro-6-methyl-pyrimidin-4-yl)-morpholine; To a solution of 2,4-dichloro-6-methylpyrimidine (998 mg, 6.0 mmol) in tetrahydrofurane (30 mL) was added at 0° C. under stirring N,N-diisopropyl ethyl amine (853 mg, 6.6 mmol) and morpholine (627 mg, 6.6 mmol) and the reaction was stirred over night at 20° C. The solvent was evaporated under reduced pressure and the residue was taken up in water and extracted twice with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered and evaporated under reduced pressure to dryness. The residue was purified by column chromatography on silica gel using heptane/ethyl acetate (7:3 v/v) as eluent to yield the title compound (893 mg, 70%) as white crystals. MS ISP (m/e): 214.1/216.2 (100/34) [(M+H)+]. 1H NMR (DMSO-D6, 300 MHz): δ (ppm)=6.73 (s, 1H), 3.65 (m, 4H), 3.58 (m, 2H), 2.25 (s, 3H).In addition 4-(2-chloro-6-methyl-pyrimidin-4-yl)-morpholine (304 mg, 24%) was obtained as white crystals. MS ISP (m/e): 214.2/216.2 (100/39) [(M+H)+]. 1H NMR (DMSO-D6, 300 MHz): δ (ppm)=6.68 (s, 1H), 3.65 (m, 8H), 2.28 (s, 3H).
References:
Baumann, Karlheinz;Flohr, Alexander;Goetschi, Erwin;Jacobsen, Helmut;Jolidon, Synese;Luebbers, Thomas US2009/215759, 2009, A1 Location in patent:Page/Page column 49
626-48-2
383 suppliers
$10.00/25 g

52026-43-4
23 suppliers
inquiry