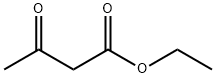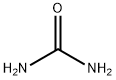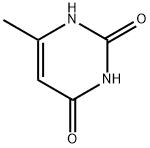
6-Methyluracil synthesis
- Product Name:6-Methyluracil
- CAS Number:626-48-2
- Molecular formula:C5H6N2O2
- Molecular Weight:126.11
Yield:-
Reaction Conditions:
with hydrogenchloride in ethanol
Steps:
1 Comparative example 1
Weigh 36g (0.6mol) of urea, 26g (0.2mol) of ethyl acetoacetate, then added 200ml of ethanol-hydrochloric acid solution (30% hydrochloric acid: 95% ethanol = 1:4), After stirring, it was dried and dehydrated and then heated to 95°C by adding sodium hydroxide solution. Then cool down to 75 °C, added hydrochloric acid to adjust ΡΗ = 1, cooling filtration, and the filtered 6-methyluracil was washed with water; Nitric acid was added to the reaction kettle, cooled to below 10 DEG C, stirred, 6-methyluracil was added, the temperature was raised to 30°C and maintained for 1 hour to filter the nitro whey; The Sodium dithionite was added into the water, stirred and dissolved, and the nitro whey liquid was added. The temperature was controlled at 35° C, and the temperature was kept for 30 minutes. After the addition of hydrochloric acid it was allowed to stand 3 h, stirred for 2 h, then subjected to filtration and drying, and amino whey was obtained; Reweigh the urea 36g and the amino whey solution into the reactor, stirred, and heated to 100°C and maintained for 20 minutes. the reaction Cool down to 90°C and added 2mol/L sodium hydroxide solution, raised the temperature to 100°C, and maintained to dissolve for 1h. the reaction was cool down to 40°C, filtrated to obtain tetrahydroxypyrimidino-[4,5d]pyrimidine sodium salt, added water, incubated at 60°C for 30 min, hydrochloric acid was added to adjust pH=4, then cooled to 15°C, filtration, washed and dried 2, 4,6,8-tetrahydroxypyrimidino-[4,5d]pyrimidine; The reactor was charged with 2,4,6,8-Tetrahydroxy-Pyrimido-(5,4D)Pyrimidine, phosphorus oxychloride, and phosphorus trichloride, stirred, and the same chlorine gas at 10 °C, heated to 110 °C reflux 24h, cooled to 15 °C, filtered, washed with water, dried to obtain 2,4, 6,8-tetrachloropyrimidine-[4,5d]pyrimidine; Acetone and tetrachloride were successively added and incubated at 20° C for 30 min. Piperidine-acetone mixed solution was added dropwise. The mixture was incubated at 30° C for 1 h, stirred with water for 1 h, filtered and dried to give 2,6-dichloro-4,8,- dipiperidine-pyrimidine; Diethanolamine 63g and 2,6-dichloro-4,8,- dipiperidine-pyrimidine were weighed and mixed, heated to 200°C for 15min, cooled to below 25°C and stirred with acetone for 30min, then incubated at 30°C for 4h, filtered and dried to obtain dipyridamole crude. The refined dipyridamole finished product 13.53, purity 98.5% and with a yield of 13.21% (calculated on the basis of ethyl acetoacetate).
References:
Hunan Erkang Pharmaceutical Co., Ltd.;Shuai Fangwen;Wang Xiangfeng;Zhang Jiawei CN108069972, 2018, A Location in patent:Paragraph 0005; 0017
56-04-2
354 suppliers
$5.00/100mg

626-48-2
373 suppliers
$10.00/25 g
![2-Piperidinemethanol, 1-[4-methyl-6-(1-methylethoxy)-2-pyrimidinyl]-](/CAS/20210305/GIF/879499-39-5.gif)
879499-39-5
0 suppliers
inquiry

626-48-2
373 suppliers
$10.00/25 g
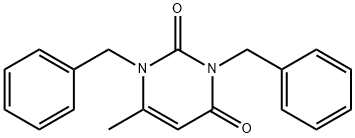
85102-59-6
2 suppliers
inquiry

626-48-2
373 suppliers
$10.00/25 g
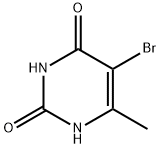
15018-56-1
108 suppliers
$26.00/250mg

626-48-2
373 suppliers
$10.00/25 g