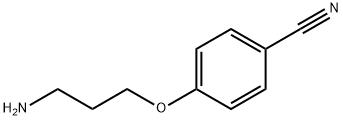
4-(3-Aminopropoxy)benzonitrile synthesis
- Product Name:4-(3-Aminopropoxy)benzonitrile
- CAS Number:116753-55-0
- Molecular formula:C10H12N2O
- Molecular Weight:176.22
![4-[3-(1,3-DIOXO-1,3-DIHYDRO-2H-ISOINDOL-2-YL)PROPOXY]BENZENECARBONITRILE](/StructureFile/ChemBookStructure21/GIF/CB5798384.gif)
313476-81-2
10 suppliers
inquiry

116753-55-0
14 suppliers
inquiry
Yield:116753-55-0 41%
Reaction Conditions:
Stage #1: 4-[3-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)propoxy]benzonitrilewith hydrazine in methanol;water; for 1 h;Heating / reflux;
Stage #2: with hydrogenchloride;water for 1.5 h;
Steps:
18.c (c) 4-(3-Aminopropoxy)benzonitrile
[0395] A mixture of 4-[3-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)propoxy]-benzonitrile (25.5 g, 83 mmol, from step (b) above) and hydrazine hydrate (4.15 g, 83 mmol) in methanol (100 mL) was refluxed for 1 h before water (120 mL) was added. The methanol was evaporated under reduced pressure and concentrated hydrochloric acid (120 mL) was added. The resulting mixture was heated on a steam bath for 1.5 h and then cooled in the refrigerator overnight. The resulting precipitate was filtered off and the filtrate was concentrated in vacuo. Water was added to the resulting residue and the solution made basic. The aqueous solution was extracted with DCM, which organic layer was then separated, dried and concentrated in vacuo to yield 6 g (41%) of the sub-title compound.
References:
US2004/229900,2004,A1 Location in patent:Page 21
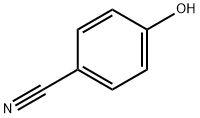
767-00-0
556 suppliers
$6.00/25g

116753-55-0
14 suppliers
inquiry
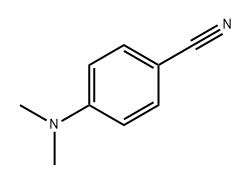
1197-19-9
130 suppliers
$31.00/5g

116753-55-0
14 suppliers
inquiry