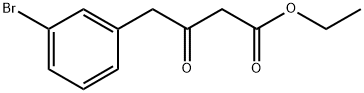
4-(3-BROMO-PHENYL)-3-OXO-BUTYRIC ACID ETHYL ESTER synthesis
- Product Name:4-(3-BROMO-PHENYL)-3-OXO-BUTYRIC ACID ETHYL ESTER
- CAS Number:866270-04-4
- Molecular formula:C12H13BrO3
- Molecular Weight:285.13
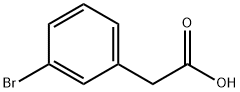
1878-67-7
326 suppliers
$5.00/5g

866270-04-4
20 suppliers
$146.66/0.5g
Yield:866270-04-4 100%
Reaction Conditions:
Stage #1: ethyl potassium malonatewith triethylamine;magnesium chloride in acetonitrile; for 3 h;
Stage #2: 3-Bromophenylacetic acidwith 1,1'-carbonyldiimidazole in acetonitrile at 18 - 25; for 18.5 h;Heating / reflux;
Steps:
87; 256
4-(3-Brornophenyi)-3-oxo-butyric acid ethyl ester (Scheme 7, A); EPO
References:
WO2006/41404,2006,A1 Location in patent:Page/Page column 84-85; 130
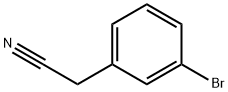
31938-07-5
235 suppliers
$8.00/5g

105-36-2
348 suppliers
$5.00/5G

866270-04-4
20 suppliers
$146.66/0.5g
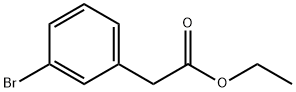
14062-30-7
66 suppliers
$6.00/1g
![Silane, (1,1-dimethylethyl)[(1-ethoxyethenyl)oxy]dimethyl-](/CAS/20200331/GIF/42201-84-3.gif)
42201-84-3
0 suppliers
inquiry

866270-04-4
20 suppliers
$146.66/0.5g
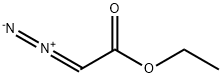
623-73-4
146 suppliers
$27.30/25ML
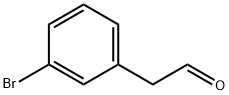
109347-40-2
50 suppliers
$45.00/10mg

866270-04-4
20 suppliers
$146.66/0.5g
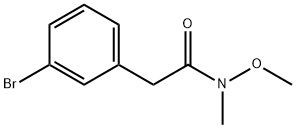
866270-03-3
0 suppliers
inquiry

866270-04-4
20 suppliers
$146.66/0.5g