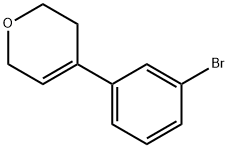
4-(3-bromophenyl)-3,6-dihydro-2H-Pyran synthesis
- Product Name:4-(3-bromophenyl)-3,6-dihydro-2H-Pyran
- CAS Number:1174324-81-2
- Molecular formula:C11H11BrO
- Molecular Weight:239.11

135048-94-1
12 suppliers
inquiry

1174324-81-2
4 suppliers
inquiry
Yield:1174324-81-2 90%
Reaction Conditions:
with toluene-4-sulfonic acid in toluene;Reflux;
Steps:
19
A mixture of 4-(3-bromophenyl)tetrahydro-2H-pyran-4-ol (11.17 g, 41.3 mmol) and p-toluenesulfonic acid monohydrate (0.797 g, 4.13 mmol) in toluene (100 mL) is heated to reflux for 30 minutes using a Dean-Stark trap to remove water. The reaction is diluted with water and 5 N NaOH and extracted with EtOAc. The organic layer is dried over Na2SO4 and the solvent is removed under reduced pressure to afford a residue that is purified on silica gel chromatography eluting with a linear gradient of 5% to 100% EtOAc in hexanes to give the title compound (8.85 g, 90%). 1H NMR (400 MHz, CDCl3) δ 2.45-2.50 (m, 2H), 3.92 (t, 2H, J=5.71 Hz), 4.31 (q, 2H, J=3.07 Hz, J=5.71 Hz), 6.12-6.14 (m, 1H), 7.19 (t, 1H, J=7.91 Hz), 7.28-7.32 (m, 1H), 7.36-7.39 (m, 1H), 7.51 (t, 1H, J=1.76 Hz).
References:
US2011/9395,2011,A1 Location in patent:Page/Page column 13
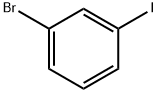
591-18-4
388 suppliers
$5.00/5g
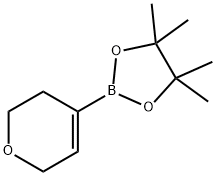
287944-16-5
218 suppliers
$7.00/250mg

1174324-81-2
4 suppliers
inquiry