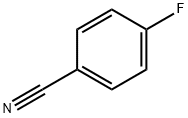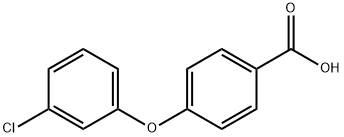
4-(3-chlorophenoxy)benzoic acid synthesis
- Product Name:4-(3-chlorophenoxy)benzoic acid
- CAS Number:1145-58-0
- Molecular formula:C13H9ClO3
- Molecular Weight:248.66
Yield:1145-58-0 99%
Reaction Conditions:
Stage #1: 3-monochlorophenol;4-fluorobenzonitrilewith potassium hydroxide in N,N-dimethyl-formamide at 175; for 0.333333 h;Sealed tube;
Stage #2: with potassium hydroxide in water; for 12 h;Reflux;
Steps:
4-(3-Chlorophenoxy)benzoic Acid (18c) was prepared by heating a mixture of KOH (199 mg, 3.0 mmol), 3-chlorophenol (337 μ, 3.2 mmol), and 4-fluorobenzonitrile (348 mg, 2.9 mmol) in DMF (1.5 mL) to 175 °C for 20 min in a sealed tube. Ether extraction gave the intermediate crude diaryl ether, which was refluxed 12 h in 30% KOH aq. The resulting solution was extracted with EtOAc, acidified, then extracted to yield the desired acid (18c) (766 mg, 99+%). NMR (CDC13) δ 8.10 (d, 2H, J= 8.7 Hz), 7.32 (t, 1H, J= 8.1 Hz), 7.21- 7.16 (m, 1H), 7.08 (t, 1H, J= 2.0 Hz), 7.04 (d, 2H, J= 8.8 Hz), 7.00-6.95 (m, 1H); MS (ESI) m/z 247.3 (M - H)-
References:
WO2013/86496,2013,A2 Location in patent:Page/Page column 33
1149-40-2
2 suppliers
inquiry

1145-58-0
23 suppliers
inquiry

1145-57-9
4 suppliers
inquiry

1145-58-0
23 suppliers
inquiry
108-43-0
294 suppliers
$13.00/5g

1145-58-0
23 suppliers
inquiry

99-90-1
468 suppliers
$6.00/25g

1145-58-0
23 suppliers
inquiry