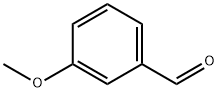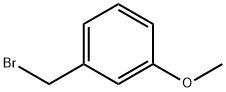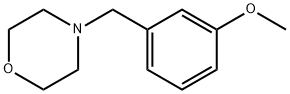
4-(3-METHOXY-BENZYL)-MORPHOLINE synthesis
- Product Name:4-(3-METHOXY-BENZYL)-MORPHOLINE
- CAS Number:122439-14-9
- Molecular formula:C12H17NO2
- Molecular Weight:207.27
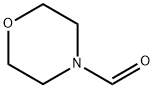
4394-85-8
303 suppliers
$6.00/25g
824-98-6
267 suppliers
$5.00/250mg

122439-14-9
11 suppliers
inquiry
Yield:122439-14-9 99%
Reaction Conditions:
with NHC-Pd(II)-Im;sodium hydroxide in water at 50; for 3 h;Inert atmosphere;Schlenk technique;
Steps:
Amination of benzyl chlorides 2 with dialkylformamides 3 in neat water catalysed by NHC-Pd(II)-Im complex 1; general procedure
General procedure: Under a N2 atmosphere, NaOH (3.0 equiv), NHC-Pd(II)-Im complex 1 (1.0 mol%), water (1.0 mL), benzyl chloride 2a (0.8 mmol), and N-formylmorpholine 3a (2.0 equiv) were successively added into a Schlenk reaction tube. The mixture was stirred at 50 °C for 3 h. After cooling to room temperature, the reaction mixture was extracted with EtOAc, washed with brine, and dried over anhydrous Na2SO4. Then the solvent was removed under reduced pressure and the residue was purified by flash column chromatography on silica gel (eluent: PE/EA = 5:1) to give the pure products 4a. 4-(3-Methoxy-benzyl)-morpholine (4c):15 Yellow liquid; 1H NMR (300 MHz): δ 7.21 (t, J = 7.8 Hz, 1H), 6.91-6.80 (m, 2H), 6.77 (dd, J = 0.9,2.4 Hz, 1H), 3.79 (s, 3H), 3.70 (t, J = 4.5 Hz, 4H), 3.46 (s, 2H), 2.43 (t,J = 4.5 Hz, 4H); 13C NMR (75 MHz): δ 159.5, 139.4, 129.1, 121.3, 114.5, 112.4, 66.9, 63.2, 55.0, 53.5.
References:
Chen, Wen-Xin;Zhang, Cai-Yun;Lu, Jian-Mei [Journal of Chemical Research,2013,vol. 37,# 10,p. 611 - 614]