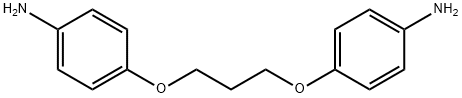
4,4'-(1,3-Propanediyl)dioxydianiline synthesis
- Product Name:4,4'-(1,3-Propanediyl)dioxydianiline
- CAS Number:52980-20-8
- Molecular formula:C15H18N2O2
- Molecular Weight:258.32
Yield:52980-20-8 77%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide in water;
Steps:
1 Preparation of Diamines Using Concentrated HCl to Hydrolyze A Diamide to Its Corresponding Diamine Salt
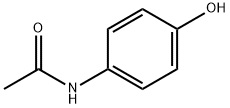
EXAMPLE 1 Preparation of Diamines Using Concentrated HCl to Hydrolyze A Diamide to Its Corresponding Diamine Salt One mole (151.7 grams) of 4-acetamidophenol was suspended in water in a five-liter, three-necked flask provided with a reflux condenser, dropping funnel, mechanical stirrer, and nitrogen inlet. After establishing a nitrogen atmosphere in the flask, one mole (40 grams) of an aqueous sodium hydroxide solution was added, with stirring, to the contents of the flask. The contents were heated, with stirring, to 80°-90 ° C., whereupon the reaction mixture in the flask turned pink. One-half of a mole (100.9 grams, 50.7 milliliters) of 1,3-dibromopropane was placed in the dropping funnel and slowly added to the flask over a period of one hour while the contents of the flask were stirred. Heating and stirring were continued for an additional four hours, during which an off-white precipitate, or intermediate product, was formed. The precipitate was later identified as 1,3-bis(4-acetamidophenoxy)propane. The intermediate product was filtered, washed with water and then recrystallized from ethanol. The recrystallized material was suspended in water in the five-liter flask, and treated with 400 milliliters of concentrated hydrochloric acid. The mixture was refluxed for five hours to hydrolyze the amide to the corresponding diamine hydrochloride salt, which remained in solution. The solution was then poured into a cold (about 0° C.) mixture of ice and aqueous sodium hydroxide to liberate the free diamine, 1,3-bis(4-aminophenoxy)propane, in 77 percent yield based on the dibromopropane. The melting point was determined to be 102°-103° C. by differential-scanning calorimetry. The yield was calculated as the number of moles of the diamine recovered per hundred moles of the dibromopropane charged to the reaction flask.
References:
US4683333,1987,A
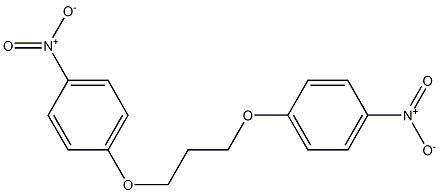
3722-79-0
8 suppliers
inquiry

52980-20-8
50 suppliers
$40.00/1g
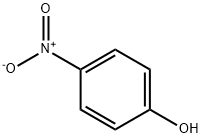
100-02-7
61 suppliers
$11.00/5G

52980-20-8
50 suppliers
$40.00/1g
![N-{4-[3-(4-acetamidophenoxy)propoxy]phenyl}acetamide](/CAS/20200401/GIF/51515-51-6.gif)
51515-51-6
3 suppliers
inquiry

52980-20-8
50 suppliers
$40.00/1g
824-78-2
33 suppliers
$14.00/1g

52980-20-8
50 suppliers
$40.00/1g