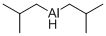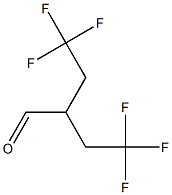
4,4,4-trifluoro-2-(2,2,2-trifluoroethyl)butanal synthesis
- Product Name:4,4,4-trifluoro-2-(2,2,2-trifluoroethyl)butanal
- CAS Number:769169-74-6
- Molecular formula:C6H6F6O
- Molecular Weight:208.1017
Yield:769169-74-6 65%
Reaction Conditions:
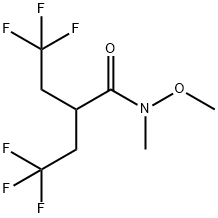
Stage #1: 4,4,4-trifluoro-N-methoxy-N-methyl-2-(2,2,2-trifluoroethyl)butyramide;diisobutylaluminium hydride in dichloromethane at -70; for 0.833333 h;
Stage #2: in dichloromethane at 25; for 0.5 h;
Steps:
2.Two.F
F. 4,4,4-Trifluoro-2-(2,2,2-trifluoro-ethyl)-butyraldehyde To a solution of 4,4,4-trifluoro-N-methoxy-N-methyl-2-(2,2,2-trifluoroethyl)-butyramide (2.67 g, 10 mmol) in CH2Cl2 (10 mL), diisobutylaluminum hydride (2.9 mL, 16 mmol) was added over 10 min at -70° C. The reaction mixture was stirred at -70° C. for 40 min, then transferred via a cannula to a flask containing 30 mL of 2N HCl at 0° C. 10 mL of conc. HCl was added and the mixture was stirred at 25° C. for 30 min. Phases were split and the aqueous phase was extracted with 5 mL of CH2Cl2. The combined organic phase was washed with brine and dried over MgSO4. NMR analysis of the solution using an internal standard indicated formation of the title product in 65% yield. The solution was used as such for further transformations.
References:
US2007/249722,2007,A1 Location in patent:Page/Page column 10-11
952714-30-6
0 suppliers
inquiry

769169-74-6
1 suppliers
inquiry
769169-73-5
2 suppliers
inquiry

769169-74-6
1 suppliers
inquiry
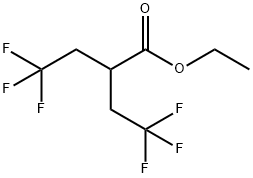
769169-72-4
0 suppliers
inquiry

769169-74-6
1 suppliers
inquiry
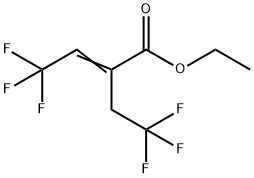
769169-71-3
0 suppliers
inquiry

769169-74-6
1 suppliers
inquiry