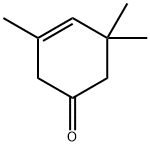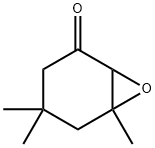
Isophorone oxide synthesis
- Product Name:Isophorone oxide
- CAS Number:10276-21-8
- Molecular formula:C9H14O2
- Molecular Weight:154.21
78-59-1
564 suppliers
$11.00/25g

10276-21-8
133 suppliers
$8.00/5g

56621-35-3
0 suppliers
inquiry
1125-21-9
273 suppliers
$8.00/5g

14203-59-9
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with tert.-butylhydroperoxide;N-hydroxyphthalimide;Cu3Al(OH)2(7+)*3.5CO3(2-) in acetonitrile at 90; for 24 h;Catalytic behavior;Reagent/catalyst;Temperature;
Steps:
Typically, the catalytic reactions were carried out in a 50 mL round flask. Cu-Al HTLcs (150 mg), NHPI (0.75 mmol), and acetonitrile (10 mL) were placed in a flask equipped with a magnetic stirrer, then the mixture was stirred vigorously for 10 min to create a finely dispersed heterogeneous system. Sequentially, isophorone (10 mmol) and 70 mass % TBHP (40 mmol) were added and the reaction mixture was refluxed. After the required time, the reaction mixture was cooled and filtered. Next, saturated NaHSO3 solution was added to the solution to remove any extra TBHP and the solution was extracted with ethyl acetate (3 × 10 mL). The organic phase was washed successively with saturated EDTA solution and saturated NaCl solution and dried overanhydrous Na2SO4. The solvent was removed by rotary evaporation and the resulting residue was analysed by GC.
References:
Zhou, Yin;Tang, Rui-Ren;Song, Dan [Chemical Papers,2016,vol. 70,# 7,p. 888 - 897]

78-59-1
564 suppliers
$11.00/25g

10276-21-8
133 suppliers
$8.00/5g

78-59-1
564 suppliers
$11.00/25g

10276-21-8
133 suppliers
$8.00/5g

56621-35-3
0 suppliers
inquiry

1125-21-9
273 suppliers
$8.00/5g