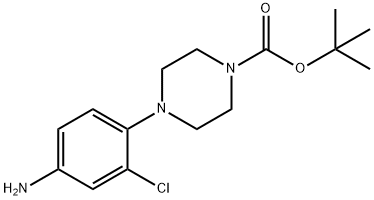
4-(4-Boc-piperazin-1-yl)-3-chloroaniline synthesis
- Product Name:4-(4-Boc-piperazin-1-yl)-3-chloroaniline
- CAS Number:193902-81-7
- Molecular formula:C15H22ClN3O2
- Molecular Weight:311.81
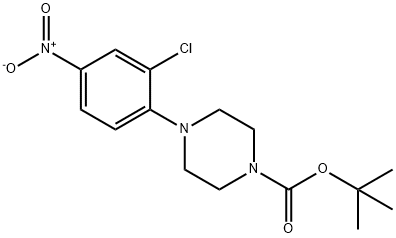
193902-80-6
13 suppliers
$24.05/250mg

193902-81-7
24 suppliers
inquiry
Yield:193902-81-7 73%
Reaction Conditions:
Stage #1: tert-butyl 4-(2-chloro-4-nitro-phenyl)piperazine-1-carboxylatewith acetic acid;zinc in tetrahydrofuran;methanol at 20; for 1 h;
Stage #2: with sodium hydrogencarbonate in tetrahydrofuran;methanol;water; for 1 h;
Steps:
221.221b
Zinc powder (1.96 g) and acetic acid (1.0 mL) were added slowly to a solution of 4-(2-chloro-4-nitro-phenyl)-piperazine-1-carboxylic acid tert-butyl ester (1.02 g) obtained in Example (221 a) in methanol (20 mL) and anhydrous tetrahydrofuran (20 mL) at room temperature. The reaction mixture was stirred for one hour, followed by addition of a saturated sodium hydrogen carbonate aqueous solution. The mixture was stirred for another hour and filtered through Celite, followed by extraction with ethyl acetate. The organic layer was washed with saturated brine and dried over sodium sulfate and concentrated. The residue was purified by column chromatography (dichloromethane:ethyl acetate 4:1). The obtained solid was vigorously stirred in hexane/isopropanol (5:1), collected by filtration and dried under reduced pressure, and 684 mg (73%) of the title compound was obtained as a pale yellow solid. 1H NMR(400MHz,CDCl3):δ(ppm)=6.86(1H, d, J=8.6Hz), 6.61(1H, d, J=2.8Hz), 6.46(1H, dd, J=8.6 and 2.8Hz), 5.06(2H, s), 3.41(4H, t, J=4.9Hz), 2.73(4H, t, J=4.9Hz), 1.41(9H, s).
References:
EP1764360,2007,A1 Location in patent:Page/Page column 161
350-30-1
317 suppliers
$14.00/1g

193902-81-7
24 suppliers
inquiry

57260-71-6
732 suppliers
$5.00/5g

193902-81-7
24 suppliers
inquiry
29682-39-1
118 suppliers
$9.00/1g

57260-71-6
732 suppliers
$5.00/5g

193902-81-7
24 suppliers
inquiry