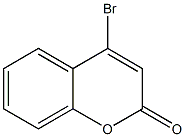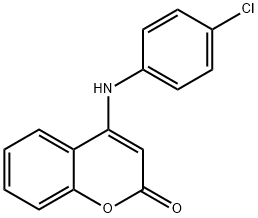
4-(4-CHLOROANILINO)-2H-CHROMEN-2-ONE synthesis
- Product Name:4-(4-CHLOROANILINO)-2H-CHROMEN-2-ONE
- CAS Number:24526-89-4
- Molecular formula:C15H10ClNO2
- Molecular Weight:271.7
Yield:24526-89-4 99%
Reaction Conditions:
in water at 100; for 0.25 h;Microwave irradiation;Reagent/catalyst;Solvent;Temperature;
Steps:
4-(Phenylamino)-2H-chromen-2-one (3a); Typical Procedures for Methods A, B
General procedure: Method A: 4-Bromocoumarin (1, 0.5 g, 2.22 mmol), aniline (2a, 0.81mL, 0.828 g, 8.89 mmol), and water (5 mL) were added to a flask suitable for a microwave oven. The mixture was irradiated at 100 °C for 15 min. The resulted solution was acidified with 1 M HCl. The precipitated solid was filtered under vacuum, washed with water (2 × 5 mL)and Et2O (2 × 5 mL) and dried to afford compound 3a (0.521 g, 99%). Method B: In a 20-mL round-bottom flask were placed 4-bromocoumarin (1, 0.2 g, 0.89 mmol) in toluene (2 mL) under an argon atmosphere. Aniline (2a, 0.09 mL, 92 mg, 0.99 mmol), Pd(OAc)2 (6 mg,0.027 mmol), PPh3 (14 mg, 0.054 mmol), and K2CO3 (0.368 g, 2.67mmol) were then added and the mixture was heated at 80 °C for 30 min. After cooling, it was filtered and washed with 1 M HCl (3 × 5 mL), water (2 × 5 mL), and Et2O (5 mL) and dried to give 3a (0.208 g, 99%)as a white solid; mp 267-268 °C (EtOH) (Lit.14a 268 °C).
References:
Balalas, Thomas;Abdul-Sada, Alaa;Hadjipavlou-Litina, Dimitra J.;Litinas, Konstantinos E. [Synthesis,2017,vol. 49,# 11,art. no. SS-2016-Z0864-P,p. 2575 - 2583]
1076-38-6
562 suppliers
$24.00/100g

106-47-8
483 suppliers
$5.00/5G

24526-89-4
19 suppliers
$118.00/500mg
17831-88-8
35 suppliers
inquiry

106-47-8
483 suppliers
$5.00/5G

24526-89-4
19 suppliers
$118.00/500mg

1076-38-6
562 suppliers
$24.00/100g

24526-89-4
19 suppliers
$118.00/500mg

108-95-2
785 suppliers
$14.00/25g

24526-89-4
19 suppliers
$118.00/500mg