
4,4-DIMETHYL-2-(2-OXOPROPYL)CYCLOHEXAN-1-ONE synthesis
- Product Name:4,4-DIMETHYL-2-(2-OXOPROPYL)CYCLOHEXAN-1-ONE
- CAS Number:163128-46-9
- Molecular formula:C11H18O2
- Molecular Weight:182.26

105104-40-3
67 suppliers
$87.00/1g
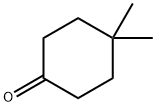
4255-62-3
200 suppliers
$10.00/1g

163128-46-9
5 suppliers
inquiry
Yield:163128-46-9 39%
Reaction Conditions:
Stage #1: 4,4-dimethylcyclohexane-1-onewith sodium hydride in toluene;mineral oil at 120; for 3 h;
Stage #2: Okahara's reagent in toluene;mineral oil;
Steps:
1.1A
EXAMPLES2Et1 2 3Scheme 1[0153] Scheme 1: Preparation of ethyl 2-(2,5,5-trimethyl-4,5,6,7-tetrahydro-lH- indol-l-yl)acetate (3).[0154] Scheme IA: Preparation of 4,4-Dimethyl-2-(2-oxopropyl)cyclohexanone (2).Sodium hydride (2.61 g, 65.4 mmol, 60% dispersion in mineral oil) was diluted in anhydrous toluene (20 mL) and stirred for 5 min, then allowed to settle, after which the supernatant was removed via cannula. The slurry was rediluted in toluene (30 mL) followed by the addition of a solution of 4,4-dimethylcyclohexanone (1) (7.50 g, 59.4 mmol) in toluene (30 mL). The reaction was heated to 120 0C for 3 h, after which neat 3-chloro-2-(methoxymethoxy)prop-l-ene (8.93 g, 65.4 mmol) was added. After heating to reflux for an additional 12 h, the reaction was cooled to room temperature, quenched by the addition of methanol (3 mL), extracted with dichloromethane (3 X 50 mL), dried (sodium sulfate), filtered, and concentrated to a brown residue which was reconstituted in dioxane (20 mL) and water (20 mL). Sulfuric acid (0.12 mL, 2.2 mmol) was added, and the resulting solution was refluxed for 2.5 h at 100 0C then cooled to room temperature, extracted with dichloromethane (3 x 100 mL), dried Docket No. 14865/176(sodium sulfate), filtered, and concentrated to a brown oil. Purification by silica gel chromatography (Luknova 120 g, 20 mL/min) using 10 to70% ethyl acetate in hexanes (over 40 min) afforded the product, 4,4-dimethyl-2-(2-oxopropyl)cyclohexanone (2) (2.5 g, 13.8 mmol). Later fractions were also collected, and were repurified using the same chromatography conditions, but using a Luknova 80 g silica column with a 20 mL/min flow rate. From this second purification, additional 4,4-dimethyl-2-(2- oxopropyl)cyclohexanone (1.70 g, 9.30 mmol) was isolated as a gold oil (total yield 4.2 g, 23.1 mmol, 39%). 1H NMR (400 MHz, CDCl3) δ (ppm): 3.06-3.15 (m, IH), 2.91 (dd, IH, J = 17.6 Hz, J = 7.6 Hz), 2.52 (app. td, IH, J = 14.4 Hz, J = 6.0 Hz), 2.24 (m, IH), 2.20 (s, 3H), 2.04-2.10 (m, IH), 1.67-1.75 (m, 2H), 1.58-1.64 (m, IH), 1.36 (dd, IH, J = 13.6 Hz, J = 13.2 Hz), 1.24 (s, 3H), 0.99 (s, 3H).
References:
WO2010/39982,2010,A1 Location in patent:Page/Page column 80-81

5020-09-7
0 suppliers
inquiry

163128-46-9
5 suppliers
inquiry
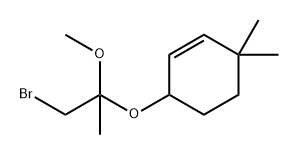
632322-77-1
0 suppliers
inquiry

163128-46-9
5 suppliers
inquiry