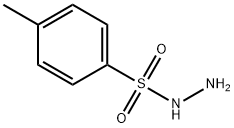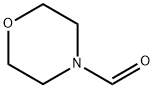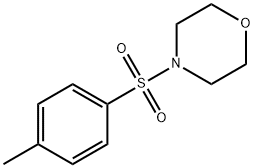
4-[(4-methylphenyl)sulphonyl]morpholine synthesis
- Product Name:4-[(4-methylphenyl)sulphonyl]morpholine
- CAS Number:6339-26-0
- Molecular formula:C11H15NO3S
- Molecular Weight:241.31
Yield:6339-26-0 99%
Reaction Conditions:
with tert.-butylhydroperoxide;iodine in water at 20; for 0.0166667 h;Concentration;Reagent/catalyst;Solvent;
Steps:
General procedure for the synthesis of sulfonamides 8-12
To a mixture of arylsulfonyl hydrazide 1 (0.5 mmol) and amine (0.75 mmol) was added TBHP (0.2 mL, 70% wt/wt in H2O, 2.0 mmol) followed by molecular iodine (0.025 g, 0.10 mmol, 20 mol%) at room temperature. The reaction was completed after the addition of iodine within a minute. After completion of the reaction, as indicated by TLC, the reaction mixture was diluted with ethyl acetate, and quenched with saturated sodium thiosulphate solution and extracted twice with ethyl acetate (2 × 10 mL). The organic layer was washed with water and dried over anhyd. sodium sulphate. The solvent was evaporated in vacuo to afford pure sulfonamide derivative (8-12). 4-(4-Tosyl)morpholine (8a): Yield: 0.120 g (>99%) as white solid; Mp: 148-149 °C; 1H NMR (500 MHz, CDCl3): δ 7.63(d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.0 Hz, 2H), 3.72 (t, J = 5.0 Hz, 4H), 2.97 (t, J = 5.0 Hz, 4H),2.43 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 143.8 (C), 131.8 (C), 129.6 (CH), 127.7(CH), 65.9 (CH2), 45.8 (CH2), 21.3 (CH3) ppm.
References:
Parumala, Santosh Kumar Reddy;Peddinti, Rama Krishna [Tetrahedron Letters,2016,vol. 57,# 11,p. 1232 - 1235] Location in patent:supporting information