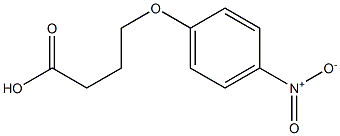
4-(4-Nitrophenoxy)Butanoic Acid synthesis
- Product Name:4-(4-Nitrophenoxy)Butanoic Acid
- CAS Number:28341-54-0
- Molecular formula:C10H11NO5
- Molecular Weight:225.198
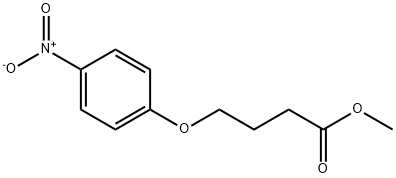
28341-53-9
18 suppliers
inquiry

28341-54-0
15 suppliers
inquiry
Yield:28341-54-0 64.3%
Reaction Conditions:
with sodium hydroxide in methanol;dichloromethane at 20;
Steps:
1.1 Step 1: Synthesis of 4-(4-nitrophenoxy)butanoic acid
In a 100ml 1-neck round flask, 1.5 g (8.29 mmol) of methyl-4-bromobutyrate, 1.15 g (8.29 mmol) of 4-nitrophenol, 1.37 g (9.94 mmol) of potassium carbonate, 100 mg of potassium iodide, and 50 ml of acetone were added. Each is added in turn, and reflux stirring is performed for at least 72 hours. The completion of the reaction was confirmed by TLC, eluent EA: n-hexane (1:4). When the completion of the reaction is confirmed, the reaction solution is first filtered, and the filtrate is concentrated. Dissolve in 50 ml of EA in the concentrate, washed twice with 30 ml of water, washed once with 30 ml of saturated brine, dried over anhydrous MgSO 4 , filtered, and the filtrate is concentrated to an intermediate 2.2 g of methyl 4-(4-nitrophenoxy)butanoate was obtained. Here, the hydrolysis reaction was carried out as follows without further purification. In 200 ml of 2N NaOH MeOH:MC (9:1) solution, the mixture was stirred at room temperature for 4 hours or more to complete the hydrolysis reaction. After adjusting the pH to 1-2 with concentrated hydrochloric acid (35%) and removing the solvent with a vacuum distiller, 50 ml of water was added to the resulting solid, followed by slurry stirring at about 40° C. for 1 hour, followed by filtration. The obtained solid was dried at 55° C. for at least 8 hours to obtain 1.2 g (yield 64.3%) of a pale yellow powder.
References:
KR2022/66701,2022,A Location in patent:Paragraph 0098-0101
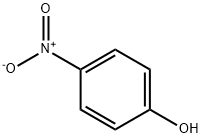
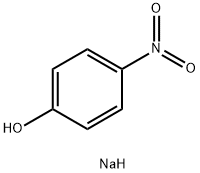
100-02-7
5 suppliers
$11.00/5G

28341-54-0
15 suppliers
inquiry
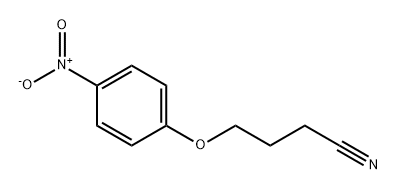
99072-20-5
0 suppliers
inquiry

28341-54-0
15 suppliers
inquiry