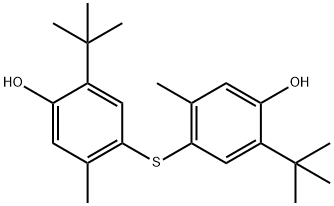
4,4'-Thiobis(6-tert-butyl-m-cresol) synthesis
1synthesis methods
- Product Name:4,4'-Thiobis(6-tert-butyl-m-cresol)
- CAS Number:96-69-5
- Molecular formula:C22H30O2S
- Molecular Weight:358.54
2-tert-butyl-5-methylphenol (164.2, 1.0mol), silver nitrate (169.9, 1.0mol), iodine (253.8g, 1.0mol) and dichloromethane 0.7L is added to the reactor, stirred at room temperature under light-proof conditions, and the colour of iodine gradually disappears, accompanied by the generation of silver iodide. After 30 min, the generated silver iodide is filtered and recovered, and the filtrate is washed with 1.1L water and recycled for washing Water. After the organic phase is dried with industrial anhydrous magnesium sulfate, Cu (8.2g), thiourea (38.1g, 0.5mol) and triethylamine 200g (about 273ml) are added inside and then reacted for 4 hours under reflux. After the reaction terminates, it is naturally cooled to room temperature, and the Cu catalyst is filtered and recovered; then water is added for dilution and washing, and after separating the aqueous phase, the organic phase is added with water again, and the pH is adjusted to 6-7 with phosphoric acid, and the aqueous phase is separated after standing. The organic phase was washed with saturated sodium chloride aqueous solution and water, respectively, dried with industrial magnesium sulfate, distilled under reduced pressure, and dichloromethane was recovered, then recrystallized with ethanol. 159.5 g of 4,4′-thiobis(6-tert-butyl-3-methylphenol) was obtained.
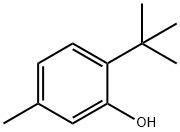
88-60-8
224 suppliers
$10.00/25g

96-69-5
290 suppliers
$9.00/25g
Yield:-
Reaction Conditions:
with hexane;sulfur dichloride
References:
Binder et al. [Journal of the American Chemical Society,1959,vol. 81,p. 3608]