
4-(5-BROMOTHIAZOL-2-YLOXY)PHENOL synthesis
- Product Name:4-(5-BROMOTHIAZOL-2-YLOXY)PHENOL
- CAS Number:904961-21-3
- Molecular formula:C9H6BrNO2S
- Molecular Weight:272.12

4175-78-4
277 suppliers
$10.00/1g
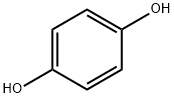
123-31-9
825 suppliers
$13.00/25g

904961-21-3
9 suppliers
$301.00/1g
Yield:904961-21-3 76%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 180; for 0.166667 h;Product distribution / selectivity;Microwave irradiation;
Steps:
11A
EXAMPLE 11; N-(3-{2-[4-(cyclopentylmethoxy)phenoxy]-1,3-thiazol-5-yl}-1-methylprop-2-yl)acetamide; EXAMPLE 11A; 4-[(5-bromo-1,3-thiazol-2-yl)oxy]phenol; A mixture of hydroquinone (440 mg, 4.0 mmol), 2,5-dibromothiazole (486 mg, 2.0 mmol) and potassium carbonate (276 mg, 2.0 mmol) in DMF (4 mL) was heated in a microwave oven at 180° C. for 10 minutes. The mixture was partitioned between ethyl acetate and saturated NaHCO3 (30 mL, 1:1). The organic phase was washed with brine (×3), dried (MgSO4), filtered and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel column with ethyl acetate/hexane (1/2) to provide 408 mg titled compound (yield 76%).
References:
US2006/178400,2006,A1 Location in patent:Page/Page column 24

4175-78-4
277 suppliers
$10.00/1g
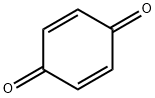
106-51-4
479 suppliers
$10.00/5g

904961-21-3
9 suppliers
$301.00/1g