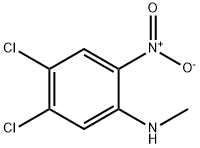
(4,5-Dichloro-2-nitro-phenyl)-methyl-amine synthesis
- Product Name:(4,5-Dichloro-2-nitro-phenyl)-methyl-amine
- CAS Number:107342-18-7
- Molecular formula:C7H6Cl2N2O2
- Molecular Weight:221.04
Yield: 89%
Reaction Conditions:
with tetrabutylammomium bromide;sodium hydroxide in toluene at 20; for 5 h;
Steps:
13-1.1 Step 1 : 4,5-dichloro-N-methyl-2-nitrobenzenamine
A solution of 4,5-dichloro-2-nitroaniline (2.00 g, 9.66 mmol), dimethyl sulfate (1.4 g, 11.1 mmol), tetrabutylammonium bromide (0.2 g, 0.58 mmol), and sodium hydroxide(4.00 g, 100.0 mmol) in toluene (30 mL) stirred for 5 h at room temperature. The reaction mixture was diluted with saturated aqueous ammonium chloride solution and extracted with 3x50 mL of ethyl acetate. The combined organic phases were dried over anhydrous sodium sulfate, filtered, and concentrated under vacuum. The residue was purified via column chromatography with silica gel (eluting with ethyl acetate/petroleum ether (1 :3)) to give 4,5- dichloro-N-methyl-2-nitrobenzenamine (1.9 g, 89%) as a white solid. MS: (ESI, m/z): 219[M- H]-.
References:
FORMA THERAPEUTICS, INC.;H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC.;LEE, Jennifer;BURNETTE, Pearlie;CHELLAPPAN, Srikumar;BARCZAK, Nicholas;CONTI, Chiara;ESCOBEDO, Jaime A.;HAN, Bingsong;LANCIA, David R., Jr.;LIU, Cuixian;MARTIN, Matthew W.;NG, Pui Yee;RUDNITSKAYA, Aleksandra;THOMASON, Jennifer R.;ZHENG, Xiaozhang WO2018/75959, 2018, A1 Location in patent:Paragraph 0513-0514

89-69-0
181 suppliers
$10.00/5g

107342-18-7
8 suppliers
inquiry




![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)