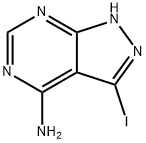
3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine synthesis
- Product Name:3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- CAS Number:151266-23-8
- Molecular formula:C5H4IN5
- Molecular Weight:261.02
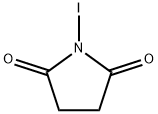
516-12-1
535 suppliers
$5.00/5g
![4-Aminopyrazolo[3,4-d]pyrimidine](/CAS/GIF/2380-63-4.gif)
2380-63-4
362 suppliers
$9.00/1g
![3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/GIF/151266-23-8.gif)
151266-23-8
329 suppliers
$22.00/1g
Yield:151266-23-8 86%
Reaction Conditions:
in N,N-dimethyl-formamide at 80; for 12 h;
Steps:
1.1 synthesis of 3-iodo-1H-Pyrazolo[3, 4-d] pyrimidine-4-amine
Pyrazolo [3,4-d] pyrimidin-4-amine (15.0 g, 111.1 mmol) was dissolved in DMF (150 mL) N-iodosuccinimide (37.5 g, 166. 6 mmol) was slowly added to the reaction mixture, and the reaction was stirred at 80 ° C for 12 h. stopWater (40 mL) was added to the reaction mixture, and the mixture was suction filtered. The solid was washed with water (80 mL), ethanol (60 mL) and dried to give to a yellow solid (24. 9 g, 86%).
References:
Guangdong HEC Pharmaceutical Co., Ltd;LIU, BING;BAI, SHUN;ZHANG, YINGJUN;ZHENG, CHANGCHUN;YANG, TIPING;ZHOU, YOUBAI CN105399756, 2016, A Location in patent:Paragraph 0155; 0165-0166
![4-Aminopyrazolo[3,4-d]pyrimidine](/CAS/GIF/2380-63-4.gif)
2380-63-4
362 suppliers
$9.00/1g
![3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/GIF/151266-23-8.gif)
151266-23-8
329 suppliers
$22.00/1g
![2H-Pyrazolo[3,4-d]pyrimidin-4-amine (9CI)](/CAS/GIF/64834-00-0.gif)
64834-00-0
7 suppliers
inquiry
![3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/GIF/151266-23-8.gif)
151266-23-8
329 suppliers
$22.00/1g
![4-Chloro-1H-pyrazolo[3,4-d]pyrimidine](/CAS/GIF/5399-92-8.gif)
5399-92-8
232 suppliers
$13.43/250mgs:
![3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/GIF/151266-23-8.gif)
151266-23-8
329 suppliers
$22.00/1g