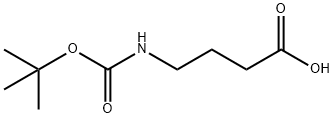
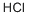4-aminobutyric acid hydrochloride synthesis
- Product Name:4-aminobutyric acid hydrochloride
- CAS Number:5959-35-3
- Molecular formula:C4H10ClNO2
- Molecular Weight:139.5807
Yield:-
Reaction Conditions:
in 1,4-dioxane;dichloromethane at 20;Inert atmosphere;
Steps:
Guanylation; General Procedure B (Table 2)
General procedure: The desired Boc-protected amino acid 4 (2.2 mmol, 1 equiv) was dissolved in CH2Cl2 (2 mL) and a solution of HCl in 1,4-dioxane (4 M, 2 mL) was added. The mixture was stirred at r.t. for 20-60 min and then the solvent was evaporated to dryness. To the resulting crude HCl acid salt (or commercial 2-Boc-2,3-diaminopropanoic acid for entries 1, 3, 5, 13, Table 2) was added CH2Cl2 (2 mL), then N,O-bis(trimethylsilyl)acetamide (2 equiv) and i-Pr2NEt (2 equiv) were added under stirring. The desired guanylating reagent 3a-g (1 equiv) was then added to the solution and the mixture was stirred for 24 h at r.t. After addition of CH2Cl2 (10 mL), the organic layer was washed with aq 1 M KHSO4 (2 × 10 mL), H2O (10 mL) and brine (10 mL), and dried (Na2SO4). The solvent was completely evaporated and the crude was rapidly purified by filtration on silica gel (2-10% CH2Cl2-MeOH) to give the guanidino acids 5a-o as white foams, which were used without further purification in the next step.
References:
Tommasi, Sara;Zanato, Chiara;Carabeo, Rey;Mangoni, Arduino A.;Dall'Angelo, Sergio;Zanda, Matteo [Synthesis,2015,vol. 47,# 19,art. no. SS-2015-N0113-OP,p. 3067 - 3078]
![Butanoic acid, 4-[(methoxycarbonyl)amino]-, methyl ester](/CAS/20200611/GIF/70288-77-6.gif)
70288-77-6
0 suppliers
inquiry

5959-35-3
6 suppliers
inquiry
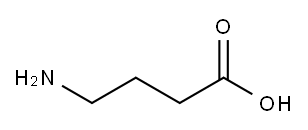
56-12-2
850 suppliers
$5.00/100mg

5959-35-3
6 suppliers
inquiry